| Identification | More | [Name]
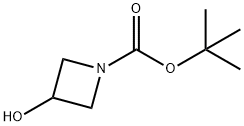
1-N-Boc-3-hydroxyazetidine | [CAS]
141699-55-0 | [Synonyms]
1-BOC-3-(HYDROXY)AZETIDINE
1-N-BOC-3-HYDROXYAZETIDINE
3-HYDROXY-AZETIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
3-HYDROXYAZETIDINE, N-BOC PROTECTED
BUTTPARK 75\04-65
N-BOC-3-HYDROXYAZETIDINE
TERT-BUTYL 3-HYDROXYAZETIDINE-1-CARBOXYLATE
1-TERT-BUTOXYCARBONYL-3-AZETIDINOL
1-TERT-BUTOXYCARBONYL-3-HYDROXYAZETIDINE
3-Hydroxyazetidine, N-BOC protected 97%
1-tert-Butoxycarbonyl-3-azetidinol
tert-butyl 3-hydroxyazetidine-1-carboxylate
1-BOC-3-(HYDROXY)AZETIDINE
1-(tert-Butoxycarbonyl)-3-azetidinol
1-(TERT-BUTOXYCARBONYL)-3-HYDROXYAZETIDINE,98.0%(GC)
1-Boc-azetidin-3-ol
1-Azetidinecarboxylic acid, 3-hydroxy-, 1,1-dimethylethyl ester | [EINECS(EC#)]
628-534-5 | [Molecular Formula]
C8H15NO3 | [MDL Number]
MFCD04115305 | [Molecular Weight]
173.21 | [MOL File]
141699-55-0.mol |
| Chemical Properties | Back Directory | [Appearance]
white powder | [Melting point ]
36-43 °C | [Boiling point ]
253.7±33.0 °C(Predicted) | [density ]
1.184±0.06 g/cm3(Predicted) | [Fp ]
>110℃ | [storage temp. ]
2-8°C | [solubility ]
soluble in Methanol | [form ]
Solid | [pka]
14.16±0.20(Predicted) | [color ]
White to light yellow | [InChI]
InChI=1S/C8H15NO3/c1-8(2,3)12-7(11)9-4-6(10)5-9/h6,10H,4-5H2,1-3H3 | [InChIKey]
XRRXRQJQQKMFBC-UHFFFAOYSA-N | [SMILES]
N1(C(OC(C)(C)C)=O)CC(O)C1 | [CAS DataBase Reference]
141699-55-0(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R41:Risk of serious damage to eyes.
R22:Harmful if swallowed. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [WGK Germany ]
3 | [TSCA ]
No | [HazardClass ]
IRRITANT | [HS Code ]
29339900 |
| Hazard Information | Back Directory | [Chemical Properties]
white powder | [Uses]
1-N-Boc-3-hydroxyazetidine is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). 1-N-Boc-3-hydroxyazetidine is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs | [Synthesis]
General procedure for the synthesis of N-Boc-3-hydroxyazetidine from 1-diphenylmethyl-3-hydroxyazetidine and di-tert-butyl dicarbonate: (1) Dissolve 1-diphenylmethyl-3-hydroxyazetidine (10.0 g, 41.8 mmol) in methanol (300 ml), add 10% palladium-carbon catalyst (10.0 g), and carry out the catalytic hydrogenation reaction at room temperature for 3 hours. After completion of the reaction, the catalyst was removed by filtration. To the filtrate was added di-tert-butyl dicarbonate (18.2 g, 83.6 mmol) and stirred at room temperature for 1 hour. After completion of the reaction, the reaction mixture was concentrated under reduced pressure. The residue was purified by silica gel column chromatography with the eluent being hexane: ethyl acetate (1:1→1:2) to afford 1-tert-butoxycarbonyl-3-hydroxyazetidine (7.05 g, 97% yield). (2) To a solution of 1-tert-butoxycarbonyl-3-hydroxyazetidine (2.5 g, 14.4 mmol) in dimethylformamide (125 ml) was added sodium hydride (55% oil dispersion) under ice bath conditions. The ice bath was stirred for 10 minutes followed by 30 minutes at room temperature. Subsequently, iodomethane (1.79 ml, 28.8 mmol) was added to the ice bath and the ice bath was stirred for 10 minutes followed by 1 hour at room temperature. After completion of the reaction, 10% aqueous acetic acid solution was added to the ice bath and stirred for 30 minutes. The reaction mixture was partitioned between ethyl acetate and 10% aqueous sodium chloride solution. The organic layer was washed sequentially with saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with hexane:ethyl acetate (2:1) as eluent to afford 1-tert-butoxycarbonyl-3-methoxyazetidine (2.18 g, 81% yield) as a colorless oil.1H-NMR (400 MHz, CDCl3): δ (ppm) 4.16-4.10 (1H, m), 4.09-4.03 (2H, m ), 3.82 (2H, dd, J = 10.2,4.4Hz), 3.28 (3H, s), 1.44 (9H, s). | [IC 50]
Non-cleavable Linker | [References]
[1] Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337. DOI:10.1038/nrd.2016.268
[2] Nalawansha DA, et al. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem Biol. 2020;27(8):998-985. DOI:10.7554/eLife.57277 |
|
|