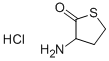
| Identification | More | [Name]
DL-Homocysteinethiolactone hydrochloride | [CAS]
6038-19-3 | [Synonyms]
DL-2-AMINO-4-MERCAPTOBUTYRIC ACID-1,4-THIOLACTONE HYDROCHLORIDE
DL-3-AMINOTETRAHYDROTHIOPHEN-2-ONE HYDROCHLORIDE
DL-HOMOCYSTEINE THIOLACTONE HCL
DL-HOMOCYSTEINE THIOLACTONE HYDROCHLORIDE
HOMOCYSTEINE THIOLACTONE HYDROCHLORIDE, DL-
TIMTEC-BB SBB004035
(+-)-dihydro-3-amino-2(3h)-thiophenonehydrochloride
d,l-homocysteinthiolaktonchlorid
dihydro-3-amino-,hydrochloride,(+-)-2(3h)-thiophenon
hctlhydrochloride
DL-homocysteine thiolactone Hydrocholoride
DL-HOMOCYSTEIN THIOLACTONE HCL
�3-Aminodihydro-2-(3H)-thiophenone Hydrochlorid
(-3-Amino-dihydro-thiophen-2-one Hydrochloride
HOMOCYSTEINE THIOLACTONE HCL, DL-(RG)
DL-HCT
DL-HOMOCYSTEINE THIOLACTONE HYDROCHLORIDE 99%
Homocysteine thiolactone hydrochloride
3-Aminodihydro-2-(3H)-thiophenone Hydrochloride
2(3H)-Thiophenone, dihydro-3-amino-, hydrochloride, (+/-) | [EINECS(EC#)]
227-923-3 | [Molecular Formula]
C4H8ClNOS | [MDL Number]
MFCD00012724 | [Molecular Weight]
153.63 | [MOL File]
6038-19-3.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Powder | [Melting point ]
202 °C (dec.)(lit.)
| [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Fine Crystalline Powder | [color ]
White | [Water Solubility ]
740.5 g/L (20 C) | [Detection Methods]
T,NMR | [Merck ]
14,4734 | [BRN ]
3562103 | [InChI]
InChI=1S/C4H7NOS.ClH/c5-3-1-2-7-4(3)6;/h3H,1-2,5H2;1H | [InChIKey]
ZSEGSUBKDDEALH-UHFFFAOYSA-N | [SMILES]
C1(N)CCSC1=O.Cl | [CAS DataBase Reference]
6038-19-3(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive;Light sensitive;Air sensitive |
| Hazard Information | Back Directory | [Chemical Properties]
White Crystalline Powder | [Uses]
DL-Homocysteine thiolactone hydrochloride is used as an intermediate of erdosteine and citiolone. | [General Description]
DL-Homocysteine thiolactone hydrochloride (HTL-HCl) is a cyclic amino acid derivative that exhibits root-growth inhibitory activity. | [Synthesis]
The general procedure for the synthesis of DL-homocysteine thiolactone hydrochloride from DL-homocysteine was as follows: 100 g of DL-methionine was added to a 3-liter cryogenic autoclave pre-cooled to -40°C in a dry ice-acetonitrile solvent mixture, followed by the slow addition of dry ammonia until DL-methionine in the concentrated liquid ammonia was completely dissolved. At a reaction temperature of -35 to -40°C, 60 g of chopped sodium metal was added in batches and the reaction process was followed by liquid chromatography. Upon completion of the reaction, stirring was stopped, the reaction mixture was allowed to stand, and the upper clear night layer was carefully transferred to another three-necked flask. Ammonium chloride was added to quench the reaction and the reaction mixture was allowed to warm up naturally to room temperature until ammonia was completely spilled. The resulting solid was a mixture of DL-homocysteine sodium salt, sodium chloride and ammonium chloride. Treatment by a cation exchange resin to remove sodium ions and subsequent adjustment of the eluate to pH 1 with concentrated hydrochloric acid resulted in 81 g of DL-homocysteine thiolactone hydrochloride in 79% yield. | [References]
[1] Patent: CN107325073, 2017, A. Location in patent: Paragraph 0018; 0019; 0020; 0021; 0022; 0023; 0024-0034 |
|
|