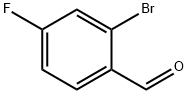
| Identification | More | [Name]
2-Bromo-4-fluorobenzaldehyde | [CAS]
59142-68-6 | [Synonyms]
2-BROMO-4-FLUOROBENZALDEHYDE | [Molecular Formula]
C7H4BrFO | [MDL Number]
MFCD00672923 | [Molecular Weight]
203.01 | [MOL File]
59142-68-6.mol |
| Chemical Properties | Back Directory | [Melting point ]
61.5 | [Boiling point ]
234.9±20.0 °C(Predicted) | [density ]
1.70 | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
powder to crystal | [color ]
White to Light yellow | [InChI]
InChI=1S/C7H4BrFO/c8-7-3-6(9)2-1-5(7)4-10/h1-4H | [InChIKey]
OPZDXMCOWFPQPE-UHFFFAOYSA-N | [SMILES]
C(=O)C1=CC=C(F)C=C1Br | [CAS DataBase Reference]
59142-68-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT, LIGHT SENSITIVE | [HS Code ]
29122990 |
| Hazard Information | Back Directory | [Chemical Properties]
White to light yellow solid | [Uses]
2-Bromo-4-fluorobenzaldehyde used as pharmaceutical intermediates.It could be the starting material to synthesise 2-Bromo-4-fluorobenzonitrile, 6-Fluoroisobenzofuran-1(3H)-one, etc. | [Synthesis]
The general procedure for the synthesis of 2-bromo-4-fluorobenzaldehyde from 2-bromo-4-fluorobenzyl alcohol was as follows: benzyl alcohol substrate (0.2 mmol), FeCl3-6H2O (0.002 mmol, 5.4 mg) and triphenylmethanol (0.2 mmol, 52 mg) were mixed in a dry reaction vessel. Subsequently, the reaction mixture was irradiated in a microwave reactor at 55 °C for 1 hour. Upon completion of the reaction, the crude product was purified by fast column chromatography to afford the target product 2-bromo-4-fluorobenzaldehyde. | [References]
[1] Tetrahedron, 2015, vol. 71, # 38, p. 6744 - 6748
[2] Synthesis (Germany), 2013, vol. 45, # 24, p. 3387 - 3391 |
|
|