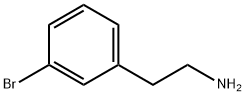
| Identification | Back Directory | [Name]
3-BROMOPHENETHYLAMINE | [CAS]
58971-11-2 | [Synonyms]
RARECHEM AL BW 0207
3-BROMOPHENETHYLAIME
3-BROMOPHENETHYLAMINE
3-broMophenylethanaMine
3-Bromobenzeneethylamine
3-Bromophenethylamine,99%
3-BROMOPHENETHYLAMINE 99%
3-BroMo-benzeneethanaMine
BenzeneethanaMine,3-broMo-
3-BROMOPHENETHYLAMINE, 98%+
2-(3-BROMOPHENYL)ETHANAMINE
3-Bromophenethylamine ,98.5%
2-(3-BROMO-PHENYL)-ETHYLAMINE
3-BroMophenethylaMine, 99% 1GR
3-BroMophenethylaMine, 99% 5GR
2-(3-Bromo-phenyl)-ethylamine hydrochloride | [EINECS(EC#)]
629-414-5 | [Molecular Formula]
C8H10BrN | [MDL Number]
MFCD01310790 | [MOL File]
58971-11-2.mol | [Molecular Weight]
200.08 |
| Chemical Properties | Back Directory | [Appearance]
clear colorless to light yellow liquid | [Boiling point ]
239-240 °C(lit.)
| [density ]
1.406 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.5740(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [form ]
Liquid | [pka]
9.83±0.10(Predicted) | [color ]
Clear colorless to light yellow |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless to light yellow liquid | [Synthesis]
General procedure for the synthesis of 3-bromophenethylamine from 3-bromocyanobenzyl: An anhydrous THF (100 mL) suspension of LiAlH4 (3.04 g, 80 mmol) was cooled to -5°C. Concentrated H2SO4 (3.9 g, 40 mmol) was added slowly dropwise and the resulting mixture was stirred for 1 h at -5°C. The reaction system was then brought to room temperature. Subsequently, a THF (5 mL) solution of 3-bromobenzeneacetonitrile (9.80 g, 50 mmol) was added slowly dropwise, and the reaction system was slowly warmed to room temperature after the dropwise addition. The reaction was continued to be stirred for 1 h at room temperature, then cooled to 0 °C and the reaction was quenched by addition of a 1:1 THF:H2O mixture (12.4 mL). Et2O (50 mL) was added, followed by 3.6 M NaOH solution (24.4 mL). The mixture was filtered through diatomaceous earth and the solids were washed well with additional Et2O. The organic phases were combined, dried over Na2SO4, filtered and concentrated in vacuum to give 3-bromophenethylamine (9.7 g, 97% yield). The crude product was used directly in the subsequent reaction.1H NMR (400 MHz, CDCl3) δ 7.38-7.30 (m, 2H), 7.20-7.10 (m, 2H), 2.96 (t, J = 7.2 Hz, 2H), 2.72 (t, J = 7.2 Hz, 2H), 1.35 (br s, 2H). m/z MS (ESI): calculated value: 199; measured value: 200/202 (M+-). | [References]
[1] Patent: WO2007/61458, 2007, A2. Location in patent: Page/Page column 44; 78
[2] Patent: WO2004/60882, 2004, A1. Location in patent: Page 29-30
[3] Patent: US2010/204214, 2010, A1. Location in patent: Page/Page column 109-110
[4] Patent: CN103880745, 2018, B. Location in patent: Paragraph 0019; 0020; 0021; 0030; 0039
[5] Patent: US2008/171754, 2008, A1. Location in patent: Page/Page column 101-102 |
|
|