| Identification | More | [Name]
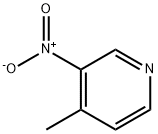
4-Methyl-3-nitropyridine | [CAS]
5832-44-0 | [Synonyms]
4-METHYL-3-NITROPYRIDINE
3-Nitro-4-methylpyridine
3-nitro-4-picoline
Pyridine, 4-methyl-3-nitro-(9CI)
Methyl-3-nitropyridine,4-
4-Methyl-3-nitropyridine,98% | [EINECS(EC#)]
628-685-7 | [Molecular Formula]
C6H6N2O2 | [MDL Number]
MFCD00051829 | [Molecular Weight]
138.12 | [MOL File]
5832-44-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Deliquescent cryst | [Melting point ]
24-28 °C | [Boiling point ]
238 °C | [density ]
1.228 g/mL at 25 °C
| [refractive index ]
n20/D 1.549
| [Fp ]
227 °F
| [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
powder to lump to clear liquid | [pka]
1.87±0.18(Predicted) | [color ]
White or Colorless to Yellow | [BRN ]
118796 | [InChI]
InChI=1S/C6H6N2O2/c1-5-2-3-7-4-6(5)8(9)10/h2-4H,1H3 | [InChIKey]
JLNRJMGYBKMDGI-UHFFFAOYSA-N | [SMILES]
C1=NC=CC(C)=C1[N+]([O-])=O | [CAS DataBase Reference]
5832-44-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R37/38:Irritating to respiratory system and skin .
R41:Risk of serious damage to eyes.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S39:Wear eye/face protection .
S36:Wear suitable protective clothing . | [RIDADR ]
2810 | [WGK Germany ]
3
| [Hazard Note ]
Harmful | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29333990 |
| Hazard Information | Back Directory | [Chemical Properties]
Deliquescent cryst | [Uses]
4-Methyl-3-nitropyridine may be used as a starting material in the synthesis of ethyl 6-azaindole-2-carboxylate and also 3-substituted azaindoles. | [General Description]
4-Methyl-3-nitropyridine, a substituted nitropyridine, can be prepared by the malonation of 4-methoxy-3-nitropyridine with sodium diethyl malonate. | [Synthesis]
The general procedure for synthesizing 3-nitro-4-methylpyridine from 4-methylpyridine is as follows:1) In step 1) of Example 1, prepare a reaction solution by adding 60 g of nitrogen pentoxide solid to 300 g of liquid sulfur dioxide at -30° C. and stirring sufficiently until completely dissolved.2) Dissolve 30 g of 4-methylpyridine in 120 g of liquid sulfur dioxide and maintain the temperature at -30° C.3 ) Slowly drop the reaction solution into 300 g of ice water for 5 minutes. ) Slowly add the nitrogen pentoxide-sulfur dioxide solution prepared in step 1) dropwise to the 4-methylpyridine solution, and react for 5 min. 4) Slowly pour the reaction solution into 300 g of ice water, and hydrolyze thoroughly. 5) Adjust the pH of the hydrolyzed solution to 8-9 using sodium carbonate. 6) Add 290 g of toluene for two extractions, and combine the extracts. 7) Remove the solvent by vacuum distillation to obtain 39 g of 3- Nitro-4-methylpyridine. The purity of the product was 99.0% by gas chromatography. | [References]
[1] Organic and Biomolecular Chemistry, 2005, vol. 3, # 3, p. 538 - 541
[2] Acta Chemica Scandinavica, 1994, vol. 48, # 12, p. 1001 - 1006
[3] Acta Chemica Scandinavica, 1994, vol. 48, # 2, p. 181 - 182
[4] Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), 1995, # 6, p. 1211 - 1216
[5] Acta Chemica Scandinavica, 1996, vol. 50, # 6, p. 556 - 557 |
|
|