| Identification | More | [Name]
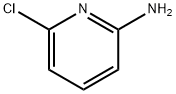
2-Amino-6-chloropyridine | [CAS]
45644-21-1 | [Synonyms]
2-AMINO-6-CHLOROPYRIDINE
2-CHLORO-6-AMINOPYRIDINE
6-AMINO-2-CHLOROPYRIDINE
6-CHLORO-2-PYRIDINAMINE
6-CHLOROPYRIDIN-2-YLAMINE
2-amino-6-chloro-pyridin
2-Pyridinamine,6-chloro-(9CI)
2-CHIORO-6-AMINOPYRIDINE
2-AMINO-6-CHLOROPYRIDINE (6-CHLOROPYRIDIN-2-YLAMINE)
6-Chloropyridine-2-amine | [EINECS(EC#)]
629-259-3 | [Molecular Formula]
C5H5ClN2 | [MDL Number]
MFCD00234068 | [Molecular Weight]
128.56 | [MOL File]
45644-21-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White to light yellow powder | [Melting point ]
69-73 °C | [Boiling point ]
255.7±20.0 °C(Predicted) | [density ]
1.326±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
Powder or Low Melting Solid | [pka]
2.76±0.24(Predicted) | [color ]
Orange to light brown | [BRN ]
108669 | [InChI]
InChI=1S/C5H5ClN2/c6-4-2-1-3-5(7)8-4/h1-3H,(H2,7,8) | [InChIKey]
OBYJTLDIQBWBHM-UHFFFAOYSA-N | [SMILES]
C1(N)=NC(Cl)=CC=C1 | [CAS DataBase Reference]
45644-21-1(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [RTECS ]
US1813350 | [Hazard Note ]
Harmful/Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29333990 |
| Hazard Information | Back Directory | [Chemical Properties]
White to light yellow powder | [Uses]
Substrate used in a Ni(II)-catalyzed homo-coupling providing diamino-2,2′-bipyridine. | [Uses]
2-Amino-6-chloropyridine used in a Ni(II)-catalyzed homo-coupling providing diamino-2,2′-bipyridine.
| [Synthesis]
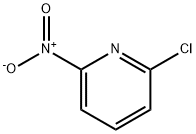
The general procedure for the synthesis of 2-amino-6-chloropyridine from 2-chloro-6-nitropyridine was as follows: the TAPEHA-Pd catalyst (0.015 g) was added to EtOH/water (1:1, 20 mL) solution containing nitroaromatics (1.0 mmol). Upon slow addition of NaBH4 (4.0 mmol), the color of the reaction mixture gradually changed to black within a few minutes, indicating the formation of palladium nanoparticles (TAPEHA-PdNPs). The reaction was stirred continuously for 1.5 h at room temperature and atmospheric pressure. After completion of the reaction, the catalyst was removed by filtration and the reaction solution was extracted with EtOAc (3 × 30 mL). The organic layers were combined, dried with MgSO4 and subsequently concentrated under vacuum. | [References]
[1] Turkish Journal of Chemistry, 2017, vol. 41, # 5, p. 784 - 792
[2] Catalysis Communications, 2015, vol. 67, p. 64 - 67 |
|
|