| Identification | More | [Name]
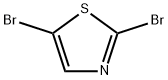
2,5-DIBROMOTHIAZOLE | [CAS]
4175-78-4 | [Synonyms]
2,5-DIBROMOTHIAZOLE
2,5-Dibromo-1,3-thiazole
2,5-DIBROMOTHIAZOLE 95% | [EINECS(EC#)]
628-983-7 | [Molecular Formula]
C3HBr2NS | [MDL Number]
MFCD00016891 | [Molecular Weight]
242.92 | [MOL File]
4175-78-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White to brown crystalline powder | [Melting point ]
45-49 °C (lit.) | [Boiling point ]
242.8±13.0 °C(Predicted) | [density ]
2.324±0.06 g/cm3(Predicted) | [Fp ]
110 °C | [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [solubility ]
soluble in Methanol | [form ]
Crystalline Powder | [pka]
-0.88±0.10(Predicted) | [color ]
White to brown | [InChI]
InChI=1S/C3HBr2NS/c4-2-1-6-3(5)7-2/h1H | [InChIKey]
XIBIQFJKUZZLLX-UHFFFAOYSA-N | [SMILES]
S1C(Br)=CN=C1Br | [CAS DataBase Reference]
4175-78-4(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [Hazard Note ]
Harmful | [HS Code ]
29341000 |
| Hazard Information | Back Directory | [Chemical Properties]
White to brown crystalline powder | [Uses]
2,5-Dibromothiazole is used in the preparation of arylthiazolylpiperidine derivatives and analogs for use as survival motor neuron (SMN) protein production modulators. | [Uses]
Used as a building block in material science.1,2 | [Synthesis]
2-Amino-5-bromothiazole (5.5 g, 30.72 mmol) was used as starting material and dissolved in acetonitrile (50 mL). Copper (II) bromide (3.43 g, 15.36 mmol) and isoamyl nitrite (4.9 mL, 36.87 mmol) were added sequentially. The reaction mixture was heated at 60 °C for 4 h with stirring. After completion of the reaction, the volatile solvent was removed by rotary evaporator. The residue was diluted with distilled water (50 mL) and subsequently extracted with ethyl acetate (25 mL x 2). The organic phases were combined, washed with saturated saline (50 mL) and dried over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (60-120 mesh, eluent 10% ethyl acetate in hexane solution) to afford the target compound 2,5-dibromothiazole (5.05 g, yield 68%) as a yellow liquid. m/z 243.6 [M+2] by LCMS. | [References]
[1] Patent: WO2015/88045, 2015, A1. Location in patent: Paragraph 0260
[2] Patent: US2017/8885, 2017, A1. Location in patent: Paragraph 0672-0673
[3] Bulletin de la Societe Chimique de France, 1962, p. 2075 - 2078
[4] Journal of Organic Chemistry, 2017, vol. 82, # 11, p. 5947 - 5951 |
|
|