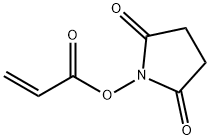
| Identification | More | [Name]
N-ACRYLOXYSUCCINIMIDE | [CAS]
38862-24-7 | [Synonyms]
1-[(1-OXO-2-PROPENYL)OXY]-2,5-PYRROLIDINEDIONE
1-(ACRYLOYLOXY)-2,5-PYRROLIDINEDIONE
ACRYLIC ACID N-HYDROXY-SUCCINIMIDE ESTER
AKOS MSC-0123
N-ACRYLOXYSUCCINIMIDE
N-Acryloxysuccinimide (NAS)
N-succinimidyl acrylate
Acrylic acid 2,5-dioxo-1-pyrrolidinyl ester
Acrylic acid 2,5-dioxopyrrolidine-1-yl ester
Acrylic acid 2,5-dioxopyrrolizino
N-(Acryloyloxy)succinimide
N-Acryloyloxysuccinimide | [Molecular Formula]
C7H7NO4 | [MDL Number]
MFCD00078261 | [Molecular Weight]
169.13 | [MOL File]
38862-24-7.mol |
| Chemical Properties | Back Directory | [Appearance]
yellow crystals and chunks | [Melting point ]
69 °C
| [Boiling point ]
298.4°C (rough estimate) | [density ]
1.4523 (rough estimate) | [refractive index ]
1.5500 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
soluble in Toluene | [form ]
Crystals and Chunks | [color ]
Yellow | [InChI]
InChI=1S/C7H7NO4/c1-2-7(11)12-8-5(9)3-4-6(8)10/h2H,1,3-4H2 | [InChIKey]
YXMISKNUHHOXFT-UHFFFAOYSA-N | [SMILES]
C(ON1C(=O)CCC1=O)(=O)C=C | [CAS DataBase Reference]
38862-24-7(CAS DataBase Reference) | [Storage Precautions]
Store under inert gas |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R40:Limited evidence of a carcinogenic effect.
R48/20/22:Harmful: danger of serious damage to health by prolonged exposure through inhalation and if swallowed . | [Safety Statements ]
S36:Wear suitable protective clothing . | [WGK Germany ]
2
| [HS Code ]
29251900 |
| Hazard Information | Back Directory | [Chemical Properties]
yellow crystals and chunks | [Uses]
sulfhydryl reactive cross-linking reagent affording sulfides and HI, can subsequently be radio-iodinated, the phenylazide function can then be photo-activated with long wavelength UV light to couple with another molecule | [Synthesis]
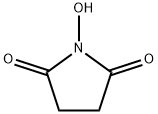
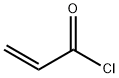
N-hydroxysuccinimide (10 g, 0.086 mol) and triethylamine (13.2 g, 0.129 mol) were dissolved in chloroform (130 mL) at 0 °C. Stirring was maintained and acryloyl chloride (8.6 g, 0.094 mol) was slowly added dropwise to the reaction mixture, the dropwise process was controlled to be completed within 2 hours. After the reaction was completed, stirring was continued at 0 °C for 30 min. Subsequently, the reaction solution was washed twice with 60 mL of saturated aqueous NaCl, and the organic phase was dried with MgSO4, filtered and concentrated to a residual volume of about 30 mL. to the concentrate was added a solvent mixture of ethyl acetate/pentane (14 mL, 1:3, v/v/v), and the mixture was kept at 0 °C to promote the crystallization of N-acryloyloxysuccinimide overnight, resulting in the target 70% yield of the Product. The structure of the product was confirmed by 1H NMR (CDCl3) with chemical shifts of 2.95 (s, 4H, CH2CH2), 6.20 (m, 1H, CH=CH2), 6.4 (m, 1H, CH=CH2) and 6.75 (m, 1H, CH=CH2), respectively. | [References]
[1] Journal of Medicinal Chemistry, 2018, vol. 61, # 10, p. 4528 - 4560
[2] Chemical Communications, 2014, vol. 50, # 95, p. 15045 - 15048
[3] Organic Letters, 2002, vol. 4, # 5, p. 737 - 740
[4] Chemical Communications, 2009, # 46, p. 7107 - 7109
[5] Journal of Medicinal Chemistry, 1995, vol. 38, # 21, p. 4179 - 4190 |
|
|