[Synthesis]
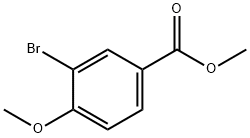
General procedure for the synthesis of 3-bromo-4-methoxybenzyl alcohol from methyl 3-bromo-4-methoxybenzoate: to a solution of methyl 3-bromo-4-methoxybenzoate (2.00 g, 8.16 mmol) in tetrahydrofuran (THF, 20 mL) at -78 °C was slowly added diisobutylaluminum hydroxide (DIBAL-H, 1.00 M in hexane, 20.4 mL ). The reaction mixture was stirred at -78 °C for 30 min, followed by warming to 0 °C and continued stirring for 30 min. Methanol (10 mL) was added dropwise to the reaction mixture at -78 °C, followed by saturated aqueous sodium potassium tartrate (Rochelle salt) (16 mL) and ethyl acetate (EtOAc, 20 mL). The mixture was stirred at room temperature for 2 h. The aqueous layer was separated and extracted with ethyl acetate. The organic layers were combined, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure. Purification by silica gel column chromatography (eluent: hexane/ethyl acetate=2:1) afforded 3-bromo-4-methoxybenzyl alcohol (1.73 g, 7.96 mmol, 98% yield) as a colorless solid; thin-layer chromatography (TLC) Rf value of 0.17 (Expanding agent: hexane/ethyl acetate=3:1); 1H NMR (300 MHz, CDCl3) δ 7.57 (1H d, J=2.2Hz, Ar-H), 7.27 (1H, dd, J=8.6,2.2Hz, Ar-H), 6.88 (1H, d, J=8.6Hz, Ar-H), 4.61 (2H, s, CH2), 3.90 (3H, s, OCH3); 13C NMR (75MHz, CDCl3) δ55.5 (OCH3 ), 134.6 (Ar-C), 132.4 (Ar-C), 112.0 (Ar-CH), 111.8 (Ar-CH), 64.4 (CH2), 56.4 (OCH3); Electron Ionization Mass Spectrometry (EIMS) m/z calculated value of C8H9BrO2 [M]+ 215.98, measured value 216.05. |