| Identification | Back Directory | [Name]
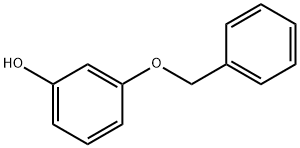
3-Benzyloxyphenol | [CAS]
3769-41-3 | [Synonyms]
3-Aenzyloxyphenol
3-(benzloxy)phenol
3-(BENZYLOXY)PHENOL
M-(BENZYLOXY)PHENOL
TIMTEC-BB SBB008506
MONOBENZYL RESOURCINOL
3-(Benzyloxy)phenol>
3-(phenylmethoxy)phenol
3-(Benzyloxy)phenol ,95%
Phenol, 3-(phenylmethoxy)-
RESORCINOL MONOBENZYL ETHER
3-(Benzyloxy)phenol, 95%, for synthesis | [Molecular Formula]
C13H12O2 | [MDL Number]
MFCD00134682 | [MOL File]
3769-41-3.mol | [Molecular Weight]
200.23 |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
3-(Benzyloxy)phenol is an isomer of benzyloxyphenol (BLP), which contains the structures 2-BLP, 3-BLP, and 4-BLP. 3-BLP is used for chromatographic identification or isomer extraction and separation studies. | [Synthesis]
General procedure for the preparation of 3-benzyloxyphenol: Resorcinol (10.0 g, 90.9 mmol) was dissolved in acetone (50 mL) and cooled to 5-10 °C. With continuous stirring, anhydrous potassium carbonate (18.8 g, 136.3 mmol) was added. Maintaining this temperature, benzyl bromide (10.88 g, 63.6 mmol) was added slowly and the cooling bath was removed after 10 minutes. The reaction mixture was refluxed for 12 to 17 hours. After the temperature was reduced to 20 to 40°C, the solid potassium carbonate was removed by filtration. The filtrate was concentrated, poured into ice water, acidified with 6N hydrochloric acid and subsequently extracted with ethyl acetate (3 x 25 mL). The organic layers were combined, washed with water, dried over anhydrous sodium sulfate and evaporated to give a brown colloidal substance. Purification by column chromatography (230-400 mesh silica gel, eluent ethyl acetate:petroleum ether = 10:90), discarding the dibenzyloxyditerpene by-product, gave pure light brown colloidal target product 3-benzyloxyphenol. Yield: 13.5 g, 68% yield. Product characterization data: 1H NMR (CDCl3, 200MHz): δ 7.50-7.30 (m, 5H), 7.12 (t, J = 8.2Hz, 1H), 6.57 (dd, J = 1.8, 6.2Hz, 1H), 6.50-6.40 (m, 2H), 5.02 (s, 2H), 4.86 (bs, 1H, D2O exchangeable). Mass spectrum (CI): m/z 201 (M++1). IR (KBr, cm-1): 3406, 3032, 1595, 1490, 1454. | [References]
[1] Patent: US2014/256657, 2014, A1. Location in patent: Paragraph 0399
[2] Tetrahedron, 2007, vol. 63, # 43, p. 10698 - 10708
[3] Journal of Organic Chemistry, 1997, vol. 62, # 10, p. 3062 - 3075
[4] Journal of Medicinal Chemistry, 2004, vol. 47, # 17, p. 4155 - 4158
[5] Chemical Communications, 2018, vol. 54, # 39, p. 4935 - 4938 |
|
|