| Identification | More | [Name]
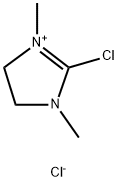
2-Chloro-1,3-dimethylimidazolidinium chloride | [CAS]
37091-73-9 | [Synonyms]
1,3-DIMETHYL-2-CHLOROIMIDAZOLINIUM CHLORIDE
2-CHLORO-1,3-DIMETHYLIMIDAZOLIDINIUM CHLORIDE
2-CHLORO-1,3-DIMETHYLIMIDAZOLINIUM CHLORIDE
DMC
Chlorodimethyllimidazoliniumchloride
2-CHLORO-1,3-DIMETHYLIMIDAZOLINIUM CHLOR
2-CHLORO-1,3-DIMETHYLIMIDAZOLIUM CHLORIDE
2-CHLORO-1,3-DIMETHYLIMIDAZOLINIUM CHLORIDE: (CA. 25% IN DICHLOROMETHANE)
2-CHLORO-1,3-DIMETHYLIMIDAZOLINIUM CHLORIDE 98+%
2-CHLORO-1,3-DIMETHYL-4,5-DIHYDRO-3H-IMIDAZOL-1-IUM CHLORIDE | [EINECS(EC#)]
629-540-0 | [Molecular Formula]
C5H12Cl2N2 | [MDL Number]
MFCD00137463 | [Molecular Weight]
171.07 | [MOL File]
37091-73-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
133-140 °C (lit.) | [storage temp. ]
2-8°C
| [solubility ]
Chloroform, Methanol, Water | [form ]
Powder or Crystalline Powder | [color ]
White to off-white | [Merck ]
13,3424 | [Stability:]
Hygroscopic | [InChI]
InChI=1S/C5H10ClN2.ClH/c1-7-3-4-8(2)5(7)6;/h3-4H2,1-2H3;1H/q+1;/p-1 | [InChIKey]
AEBBXVHGVADBHA-UHFFFAOYSA-M | [SMILES]
ClC1=[N+](CCN1C)C.[Cl-] | [CAS DataBase Reference]
37091-73-9(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
1593 | [WGK Germany ]
3
| [F ]
3-10-21 | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29332900 |
| Questions And Answer | Back Directory | [Description]
2-Chloro-1,3-dimethylimidazolidinium chloride has various applications including being used as activating agent in total synthesis of macroviracin A, cycloviracin B1 and cyclic silanes; used as reagent for synthesis of tagged glucose as an intermediate in the synthesis of branched oligosaccharides; used as fluorescent chemosensors; 1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1; used as allosteric glucokinase activators; as reactant for synthesis of organic azides from primary amines and for aza-Henry reactions1. It can act as a powerful dehydrating agent, replacing DCC under nearly neutral conditions, and has application for the construction of heterocycles2. Moreover, it can be used for one-pot synthesis of 2-aminobenzimidazoles3. It is also applicable to chlorination, oxidation, reduction, and rearrangement under nearly neutral conditions4.
| [Sources]
- https://www.sigmaaldrich.com/catalog/product/aldrich/529249?lang=en®ion=US
- https://pubs.acs.org/doi/abs/10.1021/jo990210y
- www.sciencedirect.com/science/article/pii/S004040391001395X
- J. Org. Chem., 1999, 64 (16), pp 5832–5835
|
| Hazard Information | Back Directory | [Chemical Properties]
Brown Solid | [Uses]
Reagent for synthesis of:
Tagged glucose as an intermediate in the synthesis of branched oligosaccharides
Fluorescent chemosensors
1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1
Allosteric glucokinase activators
Reactant for synthesis of:
Organic azides from primary amines
Reagent for aza-Henry reactions | [Uses]
Used in the one-pot synthesis of 2-aminobenzimidazoles. | [reaction suitability]
reaction type: Coupling Reactions | [Synthesis]
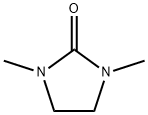
General procedure for the synthesis of 2-chloro-1,3-dimethylimidazolium chloride from 1,3-dimethyl-2-imidazolidinone: In a 1000 ml three-necked reaction flask, 1,3-dimethyl-2-imidazolidinone (34.2 g, 0.3 mol) and carbon tetrachloride (400 ml) were added. A carbon tetrachloride solution of solid phosgene (containing 30 g of solid phosgene, 0.1 mole, dissolved in 100 mL of carbon tetrachloride) was added slowly and dropwise. The temperature of the reaction mixture was controlled below 5°C with vigorous stirring for 0.5 hours. Subsequently, the reaction mixture was brought to room temperature and the reaction was continued for 1 hour. Next, it was heated to 50°C and kept at this temperature for 4 hours. Upon completion of the reaction, the mixture was cooled to room temperature, filtered, and washed with a small amount of carbon tetrachloride to afford 49 g of a pure white crystalline product, 2-chloro-1,3-dimethylimidazolium chloride, in 96.6% yield. | [References]
[1] Patent: CN105367478, 2016, A. Location in patent: Paragraph 0021; 0027; 0028
[2] European Journal of Inorganic Chemistry, 2005, # 19, p. 3815 - 3824
[3] Chemistry - A European Journal, 2016, vol. 22, # 45, p. 16187 - 16199
[4] Synthesis, 2009, # 13, p. 2267 - 2277
[5] Helvetica Chimica Acta, 1985, vol. 68, p. 1543 - 1556 |
|
|