| Identification | Back Directory | [Name]
Lurasidone hydrochloride | [CAS]
367514-88-3 | [Synonyms]
CS-864
Lurasidon hcl
lurasidone HCI
Latuda, SM 13496
urasidone HydrochL
Exodiene Lurasidone
Lurasidonhydrochlorid
Lurasidonhydrochloride
Lurasidone Hydrochlorid
urasidone hydrochloride
SM-13496 (Hydrochloride)
Lurasidone HCl (SM-13496)
Lurasidone Hydrochloride API
Lurasidone hydrochloride salt
Lurasidone Hydrochloride Tablets
Lurasidone hydrochloride solution
Lurasidone hydrochloride USP/EP/BP
Lurasidone HCl / Lurasidone Hydrochloride
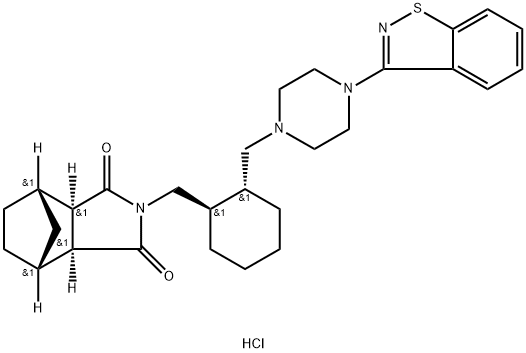
(3aR,4R,7S,7aS)-2-(((1R,2R)-2-((4-(benzo[d]isothiazol-3-yl)
(3aR,4S,7R,7aS)-2-[(1R,2R)-2-[4-(1,2-Benzisothiazol-3-yl)pip...
(3aR,4S,7R,7aS)-2-[(1R,2R)-2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-
(R)-2-[[2-(4-aminophenyl ethyl] aminol-1-phenylethanol monohydrochloride
Lurasidone hydrochloride,SM13496,Inhibitor,5-hydroxytryptamine Receptor,Lurasidone,5-HT Receptor,Dopamine Receptor,inhibit,SM 13496,Serotonin Receptor
(1R,2S,3R,4S)-N-[(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinylMethyl]-1-cyclohexylMethyl]-2,3-bicyclo[2.2.1]heptanedicarboxyiMide hydrochloride
(3aR,4S,7R,7aS)-2-[(1R,2R)-2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-ylMethyl]cyclohexylMethyl]hexahydro-1H-4,7-Methanoisoindole-1,3-dione hydrochloride
(1R,2S,6R,7S)-4-{[(1R,2R)-2-{[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]methyl}cyclohexyl]methyl}-4-azatricyclo[5.2.1.0^{2,6}]decane-3,5-dione hydrochloride
(3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl methyl] cyclohexylmethyl}hexahydro-4,7-methano-2H-isoindole-1,3-dione hydrochloride
(3aR,4S,7R,7aS)-2-(((1R,2R)-2-((4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)methyl)cyclohexyl)methyl)hexahydro-1H-4,7-methanoisoindole-1,3(2H)-dione hydrochloride
(3aR,4S,7R,7aS)-2-[[(1R,2R)-2-[[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]methyl]cyclohexyl]methyl]hexahydro-4,7-methano-1H-isoindole-1,3(2H)-dione hydrochloride
4,7-Methano-1H-isoindole-1,3(2H)-dione, 2-[[(1R,2R)-2-[[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]methyl]cyclohexyl]methyl]hexahydro-, hydrochloride (1:1), (3aR,4S,7R,7aS)-
Lurasidone HydrochlorideQ: What is
Lurasidone Hydrochloride Q: What is the CAS Number of
Lurasidone Hydrochloride Q: What is the storage condition of
Lurasidone Hydrochloride Q: What are the applications of
Lurasidone Hydrochloride | [EINECS(EC#)]
682-423-6 | [Molecular Formula]
C28H36N4O2S | [MDL Number]
MFCD18917095 | [MOL File]
367514-88-3.mol | [Molecular Weight]
492.684 |
| Chemical Properties | Back Directory | [Melting point ]
198-205°C | [Fp ]
9℃ | [storage temp. ]
Refrigerator | [solubility ]
DMSO : 10 mg/mL (18.90 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) | [form ]
Powder | [color ]
White to off-white | [InChIKey]
PQXKDMSYBGKCJA-CVTJIBDQSA-N | [SMILES]
O=C1N(C(=O)[C@@]2([H])[C@]3([H])CC[C@@]([H])(C3)[C@@]12[H])C[C@@H]1CCCC[C@H]1CN1CCN(C2=NSC3=CC=CC=C23)CC1.Cl |&1:5,7,11,14,17,22,r| |
| Hazard Information | Back Directory | [Chemical Properties]
Off-White Solid | [Uses]
An antipsychotic used for treatment of schizophrenia. | [Uses]
Lurasidone is an atypical antipsychotic, inhibits Dopamine D2, 5-HT2A, 5-HT7, 5-HT1A and noradrenaline α2C with IC50 of 1.68 nM, 2.03 nM, 0.495 nM, 6.75 nM and 10.8 nM, respectively | [Definition]
ChEBI: A hydrochloride obtained by reaction of lurasidone with one equivalent of hydrochloric acid. An atypical antipsychotic agent used for the treatment of schizophrenia. | [Biological Activity]
Lurasidone is an atypical antipsychoticused clinically for treatment of schizophrenia and bi-polar disorder. It has nanomolar activity as an antagonist at dopamine D2serotonin 5-HT2A5-HT7and α2C-adrenergic receptors and as an agonist at 5-HT1A receptors. | [Synthesis]
(3aR,7aR)-4'-(benzo[d]isothiazol-3-yl)octahydrospiro[isoindol-2,1'-piperazin]-2-ium methanesulfonate (Intermediate 4, 28.8 kg, 66.2 mol) and (3aR,4S,7R,7aS)-rel-hexahydro-1H-4,7-bridged methylidene isoindole-1,3(2H)-dione (Intermediate 5. 12.0 kg, 72.8 mol) as raw material and potassium carbonate (11.0 kg, 79.7 mol) as base were suspended in toluene (270 L). The resulting suspension was heated and refluxed at 105 °C for 15 h. The reaction process was monitored by UPLC. Upon completion of the reaction, the reaction mixture was cooled to room temperature and quenched by the addition of water (90 L). The organic and aqueous phases were separated and the organic phase was concentrated to a smaller volume. Subsequently, the concentrated organic phase was treated with hydrochloric acid in isopropanol to afford the target product (3aR,4S,7R,7aS)-2-(((1R,2R)-2-((4-(benzo[d]isothiazol-3- yl)piperazin-1- yl)methyl)cyclohexyl)methyl)hexahydro-1H-4,7-methanoisoindole-1,3(2H)-dione hydrochloride ( Lurasidone hydrochloride, 34.4 kg, yield 98.3%, HPLC purity 99.49%). | [storage]
Store at -20°C | [References]
[1] Patent: WO2015/56205, 2015, A1. Location in patent: Page/Page column 14
[2] Patent: WO2013/121440, 2013, A1 |
|
|




![(3aR,7aR)-4'-(1,2-Benzisothiazol-3-yl)octahydrospiro[2H-isoindole-2,1'-piperaziniuM] Methanesulfonate](/CAS/20180906/GIF/186204-37-5.gif)

