| Identification | More | [Name]
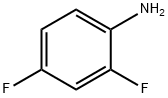
2,4-Difluoroaniline | [CAS]
367-25-9 | [Synonyms]
1-amino-2,4-difluorobenzene
2,4-DIFLUOROBENZENAMINE
2,4-DIFLUOROPHENYLAMINE
AKOS 91127
LABOTEST-BB LTBB000653
2,4-difluoro-anilin
Aniline, 2,4-difluoro-
Benzenamine, 2,4-difluoro-
Difluoroaniline
2,4-DIFLUROANILINE
2,4-Difluorobenzeneamine
2,4-Difluoroaniline,99%
2,4-Difluoroaniline97%
2,4-DIFLOUROANILINE
2,4-Difluoranilin
2,4-difluoro-benzenamin
2,4-DIFLUOROANILINE 99.5%
4,6-Difluorophenylamine | [EINECS(EC#)]
206-687-5 | [Molecular Formula]
C6H5F2N | [MDL Number]
MFCD00007648 | [Molecular Weight]
129.11 | [MOL File]
367-25-9.mol |
| Chemical Properties | Back Directory | [Appearance]
dark red liquid | [Melting point ]
-7.5 °C (lit.) | [Boiling point ]
170 °C/753 mmHg (lit.) | [density ]
1.268 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.506(lit.)
| [Fp ]
145 °F
| [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
Not miscible or difficult to mix. | [form ]
Liquid | [pka]
3.26±0.10(Predicted) | [color ]
Clear brown | [Specific Gravity]
1.284 (20/4℃) | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides. | [Water Solubility ]
1-5 g/100 mL at 20.55 ºC | [Merck ]
14,3147 | [BRN ]
2802556 | [LogP]
1.7 at 25℃ and pH5-9 | [CAS DataBase Reference]
367-25-9(CAS DataBase Reference) | [NIST Chemistry Reference]
2,4-Difluoroaniline(367-25-9) | [EPA Substance Registry System]
2,4-Difluoroaniline (367-25-9) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T,Xi | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN 2941 6.1/PG 3
| [WGK Germany ]
1
| [RTECS ]
BX3680000
| [F ]
8-9-23 | [Hazard Note ]
Toxic | [TSCA ]
T | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29214210 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Iron-->Potassium fluoride-->1,3-Dichlorobenzene-->m-Phenylenediamine-->2,4-Difluoronitrobenzene-->2-Chloro-3,4-difluoroaniline-->3-CHLORO-2,4-DIFLUOROANILINE 99-->Activated carbon-->Palladium-->Hydrogen | [Preparation Products]
TOSUFLOXACIN TOSILATE-->TEMAFLOXACIN-->Diflufenican-->Diflunisal-->2,4,5-Trifluorobenzoic acid-->N-(4-fluoro-2-Methoxy-5-nitrophenyl)-4-(1-Methylindol-3-yl)pyriMidin-2-aMine-->2-Bromo-4,6-difluorophenol-->2,4-DIFLUORO-6-NITROANILINE-->2-AMINO-4,6-DIFLUOROBENZOTHIAZOLE-->Benzamide, 5-chloro-N-(2,4-difluorophenyl)-2-hydroxy--->1H-Pyrrole-3-carboxaldehyde, 1-(2,4-difluorophenyl)-2,5-dimethyl--->1-(2,4-DIFLUORO-PHENYL)-1H-PYRROLE-2-CARBALDEHYDE-->N-(2,4-DIFLUOROPHENYL)MALEAMIC ACID-->benzyl 2,4-difluoro-3-forMylphenylcarbaMate-->Acetamide, N-(2,4-difluorophenyl)-2-(hydroxyimino)- |
| Hazard Information | Back Directory | [General Description]
Dark reddish-purple liquid. | [Reactivity Profile]
Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. | [Air & Water Reactions]
This chemical may be sensitive to prolonged exposure to air. . Water soluble. | [Health Hazard]
ACUTE/CHRONIC HAZARDS: This compound may be irritating to tissues. | [Fire Hazard]
This compound is combustible. | [Chemical Properties]
dark red liquid | [Uses]
2,4-Difluoroaniline is an important raw material and intermediate used in organic synthesis, pharmaceuticals agrochemicals and dyestuff fields. | [Synthesis]
The general procedure for the synthesis of 2,4-difluoroaniline from 2,4-difluoronitrobenzene was as follows: 2,4-difluoro-1-nitrobenzene (1 g), methanol (10 mL), and Pd/C catalyst (100 mg) were added to a 100 mL single-necked reaction flask. Subsequently, the reaction system was replaced three times with hydrogen to remove air. The reaction mixture was stirred at room temperature for 2-3 hours. Upon completion of the reaction, the catalyst was removed by filtration under reduced pressure and the filtrate was concentrated to give 810 mg of the target product 2,4-difluoroaniline. | [References]
[1] Synthetic Communications, 2012, vol. 42, # 2, p. 213 - 222
[2] Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry, 2011, vol. 41, # 1, p. 114 - 119
[3] Journal fuer Praktische Chemie (Leipzig), 1934, vol. <2> 140, p. 97,111
[4] Chemische Berichte, 1937, vol. 70, p. 2396,2400
[5] Journal fuer Praktische Chemie (Leipzig), 1934, vol. <2> 140, p. 97,111 |
| Questions And Answer | Back Directory | [Description]
2,4-difluoroaniline is an important intermediate used in organic synthesis, pharmaceuticals agrochemicals, dyes, and agricultural chemicals.
| [Reference]
R. Garth Pews, J. A. Gall, Process and intermediates for the preparation of 2,6-difluoroaniline, Paten US5041674A
|
|
|