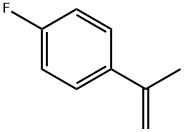
| Identification | More | [Name]
1-Fluoro-4-(1-methylethenyl)benzene | [CAS]
350-40-3 | [Synonyms]
1-FLUORO-4-(1-METHYLETHENYL)BENZENE
2-(4-FLUOROPHENYL)-1-PROPENE
2-(4-FLUOROPHENYL)PROPENE
4-FLUORO-ALPHA-METHYLSTYRENE
4-FLUORO-A-METHYLSTYRENE
1-Fluoro-4-isopropenylbenzene
4-fluoro-methylstyrene
Benzene, 1-fluoro-4-(1-methylethenyl)-
p-Fluoro-alpha-methylstyrene
Styrene, p-fluoro-alpha-methyl-
Fluoroalphamethylstyrene
P-Fluoro-alfa-methylstyrene
2-(4-Fluorophenyl)prop-1-ene
4-fluoro-à-methylstyrene
4-Fluoro-alpha-methylstyrene 97%
4-Fluoro-alpha-methylstyrene97%
4-Fluoro-ɑ-methylstyrene, 95%
4-Fluoro-alpha-methylstyrene (stabilized with TBC)
1-Fluoro-4-prop-1-en-2-ylbenzene
1-Isopropenyl-4-fluorobenzene | [EINECS(EC#)]
206-501-2 | [Molecular Formula]
C9H9F | [MDL Number]
MFCD00042297 | [Molecular Weight]
136.17 | [MOL File]
350-40-3.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R10:Flammable. | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 1993 3/PG 3
| [WGK Germany ]
3
| [Hazard Note ]
Irritant/Keep Cold | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
2942000090 |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless to yellowish liquid | [Uses]
4-Fluoro-a-methylstyrene is used in the synthesis of Paroxetine (P205750), which is a selective serotonin reuptake inhibitor. Used as an antidepressant. | [Synthesis]
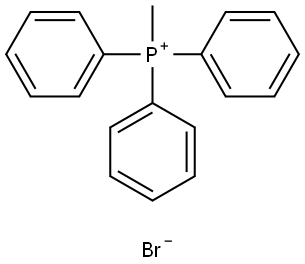
GENERAL METHOD: Potassium tert-butoxide (t-BuOK, 2.47 g, 22.00 mmol) was added to an anhydrous tetrahydrofuran (THF, 70 mL) solution of methyltriphenylphosphonium bromide (7.12 g, 20.00 mmol) in four batches at 0 °C. The reaction mixture was stirred at 0 °C for 2 h, followed by slow dropwise addition of anhydrous THF (20 mL) solution of 4-fluoroacetophenone (20 mmol) over 30 min. After the dropwise addition, the reaction mixture was continued to be stirred at room temperature for 16 hours. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride (NH4Cl, 50 mL) solution. The reaction solution was extracted three times with petroleum ether (3 x 50 mL). All organic phases were combined, washed with saturated saline (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated. Finally, the residue was purified by fast column chromatography using petroleum ether (Pet) as eluent to afford the target product 1-fluoro-4-(prop-1-en-2-yl)benzene. | [References]
[1] Tetrahedron, 2008, vol. 64, # 37, p. 8610 - 8617
[2] Organic Letters, 2018, vol. 20, # 18, p. 5747 - 5751
[3] Angewandte Chemie - International Edition, 2013, vol. 52, # 32, p. 8450 - 8453
[4] Angew. Chem., 2013, vol. 125, # 32, p. 8608 - 8611,4
[5] Tetrahedron, 2017, vol. 73, # 49, p. 6901 - 6905 |
|
|