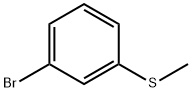
| Identification | More | [Name]
3-Bromothioanisole | [CAS]
33733-73-2 | [Synonyms]
1-BROMO-3-(METHYLTHIO)BENZENE
3-BROMOPHENYL METHYL SULFIDE
3-BROMOTHIOANISOLE
M-BROMO(METHYLTHIO)BENZENE
M-BROMOTHIOANISOLE
3-Bromophenyl methyl sulphide
3-Bromothioanisol/3-Bromophenyl methyl sulphide
m-bromothioanisole(MBTA)
2-Bromophenyl methyl sulphide
3-BROMOTHIOANISOLE/3-BROMOPHENYL METHYL SULPHIDE
3-BROMOTHIOANISOLE 98%
(3-bromophenyl)(methyl)sulfane
1-Methylthio-3-bromobenzene
3-(Methylthio)phenyl bromide
m-Bromophenyl(methyl) sulfide
Methyl[3-bromophenyl] sulfide | [EINECS(EC#)]
608-901-6 | [Molecular Formula]
C7H7BrS | [MDL Number]
MFCD00041395 | [Molecular Weight]
203.1 | [MOL File]
33733-73-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Pale-Yellow Liquid | [Boiling point ]
124-125 °C10 mm Hg(lit.) | [density ]
1.51 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.628(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
soluble in Chloroform, Ethyl Acetate | [form ]
clear liquid to slightly cloudy liquid | [color ]
Colorless to Yellow | [Specific Gravity]
1.510 | [Sensitive ]
Stench | [BRN ]
2078598 | [CAS DataBase Reference]
33733-73-2(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
36/37/38 | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
UN 3334 | [WGK Germany ]
3
| [Hazard Note ]
Irritant/Stench | [HazardClass ]
IRRITANT, STENCH | [HS Code ]
29309090 |
| Hazard Information | Back Directory | [Chemical Properties]
Pale-Yellow Liquid | [Uses]
3-Bromothioanisole is a useful reagent for the preparation of (Z)-1,2-diborylalkenes via sodium-metal-promoted reductive 1,2-syn-diboration of alkynes with reduction-??resistant trimethoxyborane. | [Synthesis]
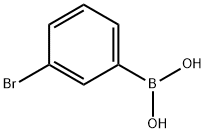
GENERAL METHOD: 3-bromophenylboronic acid (1.0 mmol), dimethyl disulfide (2.0 mmol), di-tert-butyl peroxide (DTBP, 3.0 mmol) and acetonitrile (CH3CN, 2.0 mL) were added sequentially to a sealed tube. The reaction mixture was stirred at 120 °C in air for 12 hours. After completion of the reaction, it was cooled to room temperature and the reaction mixture was diluted with deionized water (5 mL) and subsequently extracted with ethyl acetate (EtOAc, 4 × 10 mL). The organic phases were combined, washed with saturated saline (3 × 10 mL), dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated. The crude product was purified by silica gel column chromatography with ethyl acetate/hexane as eluent (v/v = 1:30 to 1:100). The resulting 3-bromothioanisole was characterized by spectroscopic analyses (NMR hydrogen spectroscopy, carbon spectroscopy, etc.) and compared with the data of known compounds (see Supporting Information for details). | [References]
[1] Synlett, 2016, vol. 27, # 15, p. 2269 - 2273 |
|
|