| Identification | Back Directory | [Name]
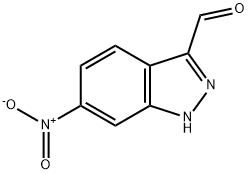
6-NITRO INDAZOLE-3-CARBOXALDEHYDE | [CAS]
315203-37-3 | [Synonyms]
6-NITRO-3-CARBOXALDEHYDE
6-NITRO INDAZOLE-3-CARBOXALDEHYD
6-NITRO INDAZOLE-3-CARBOXALDEHYDE
6-NITRO-1H-INDAZOLE-3-CARBALDEHYDE
6-nitro-2H-indazole-3-carbaldehyde
6-nitro-2H-indazole-3-carboxaldehyde
6-NITRO-1H-INDAZOLE-3-CARBOXALDEHYDE
6-Nitro-1H-indazole-3-carboxyaldehyde
1H-Indazole-3-carboxaldehyde, 6-nitro- | [Molecular Formula]
C8H5N3O3 | [MDL Number]
MFCD03093357 | [MOL File]
315203-37-3.mol | [Molecular Weight]
191.14 |
| Hazard Information | Back Directory | [Synthesis]
The general procedure for the synthesis of 6-nitro-indazole-3-carboxaldehyde from 6-nitroindole was as follows: in a 500 mL round-bottomed flask equipped with a stirrer, sodium nitrite (NaNO2, 6.38 g, 92.5 mmol) and distilled water (150 mL) were added sequentially. 6-Nitroindole (5.15 g, 31.7 mmol) was slowly added to the above solution at 20°C, followed by vigorous stirring of the reaction mixture until a homogeneous suspension was formed (about 5 min). 6 M hydrochloric acid (HCl, 14 mL) was added dropwise to this bright yellow suspension through the addition funnel over 30 min. After the dropwise addition, the reaction temperature was maintained at 20°C and the reaction mixture continued to be stirred for 90 minutes. During the reaction, a small sample of the reaction solution was taken, filtered, and the precipitate was dissolved in a minimal amount of high-performance liquid chromatography (HPLC)-grade acetonitrile (MeCN) and analyzed by liquid chromatography-mass spectrometry (LC-MS) to confirm completion of the reaction. Upon completion of the reaction, the reaction mixture was vacuum filtered, the precipitate was collected, and the precipitate was washed with additional distilled water (50 mL). The washed precipitate was dried to afford the target product 6-nitro-indazole-3-carbaldehyde (1.37 g, 77% yield) as an orange solid. Melting point: 120-160°C (color changed from orange to brown), decomposed above 200°C; LC/MS retention time (tR) = 2.40 min (Method A); mass-to-charge ratio (m/z) = 190.05 (MH+); 1H NMR (400 MHz, DMSO-d6) δ = 10.22 (s, 1H), 8.57 (d, J = 1.5 Hz , 1H), 8.29 (dd, J = 0.5, 8.9 Hz, 1H), 8.13 (dd, J = 2.0, 8.9 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ = 187.7, 146.9, 124.0, 122.4, 118.7, 108.6. | [References]
[1] Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 9, p. 2514 - 2529
[2] RSC Advances, 2018, vol. 8, # 24, p. 13121 - 13128
[3] Journal of Medicinal Chemistry, 2001, vol. 44, # 7, p. 1021 - 1024 |
|
| Company Name: |
China Langchem Inc.
|
| Tel: |
0086-21-58956006 |
| Website: |
www.yuhua99.com/ShowSupplierProductsList19141/0_EN.htm |
|