| Identification | More | [Name]
Rosiglitazone hydrochloride | [CAS]
302543-62-0 | [Synonyms]
ROSIGLITAZONE HYDROCHLORIDE 99%
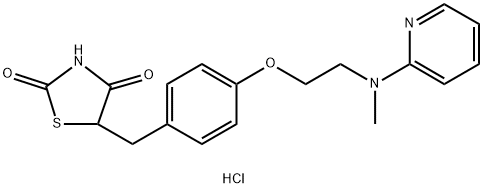
5-[4-[2-[N-Methyl-N-(pyridinyl) amino]ethoxy]benzyl]thiazolidine-2,4-dione hydrochloride
Rosiglitazone hydrochloride | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C18H19N3O3S.HCl | [MDL Number]
MFCD04112701 | [Molecular Weight]
393.89 | [MOL File]
302543-62-0.mol |
| Chemical Properties | Back Directory | [storage temp. ]
Store at -20°C | [solubility ]
≥39.4 mg/mL in DMSO; ≥2.81 mg/mL in H2O with gentle warming and ultrasonic; ≥51.2 mg/mL in EtOH with ultrasonic | [form ]
solid | [color ]
White to off-white | [InChI]
InChI=1S/C18H19N3O3S.ClH/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15;/h2-9,15H,10-12H2,1H3,(H,20,22,23);1H | [InChIKey]
XRSCTTPDKURIIJ-UHFFFAOYSA-N | [SMILES]
C(C1C=CC(OCCN(C2N=CC=CC=2)C)=CC=1)C1C(NC(=O)S1)=O.Cl | [CAS DataBase Reference]
302543-62-0(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Rosiglitazone is a hypoglycemic drug developed by GS, and the revalent compounds are disclosed in European Patent EP 306228. | [Uses]
Rosiglitazone hydrochloride has better stability than rosiglitazone maleate, and is very suitable for application in the manufacture of common pharmaceutical preparations. It is a thiazolidinedione antidiabetic drug, which can effectively control blood sugar by improving insulin sensitivity. | [Biological Activity]
rosiglitazone is a blood glucose-lowering drugs, stimulating insulin secretion by binding to the ppar receptors in fat cells. | [in vivo]
Rosiglitazone hydrochloride (oral administration, 5 mg/kg, daily for 8 weeks) decreases the serum glucose in diabetic rats[5].
Rosiglitazone hydrochloride (intraperitoneal injection, 3 mg/kg/day) ameliorates airway inflammation induced by cigarette smoke via inhibiting the M1 macrophage polarization by activating PPARγ and RXRα in male Wistar rats[6].
Rosiglitazone hydrochloride (intraperitoneal injection, 10 mg/kg, once every 2 days) inhibits subcutaneous ovarian cancer growth in A2780 and SKOV3 mouse subcutaneous xenograft models[7]. | Animal Model: | Streptozotocin (STZ)-induced diabetic rats[5] | | Dosage: | 5 mg/kg | | Administration: | Oral administration, daily for 8 weeks. | | Result: | Decreased IL-6, TNF-α, and VCAM-1 levels in diabetic group.
Displayed lower levels of lipid peroxidation and NOx with an increase in aortic GSH and SOD levels compared to diabetic groups.
|
| Animal Model: | Male Wistar rats[6] | | Dosage: | 3 mg/kg/day | | Administration: | Intraperitoneal injection, twice a day, 6 days per week for 12 consecutive weeks | | Result: | Ameliorated emphysema, elevated PEF, and higher level of total cells, neutrophils and cytokines (TNF-α and IL-1β) induced by cigarette smoke (CS).
Inhibited CS-induced M1 macrophage polarization and decreased the ratio of M1/M2.
|
| [target]
| [IC 50]
PPARγ: 40 nM (Kd); PPARγ: 60 nM (EC50); TRPC5: 30 μM (EC50); TRPM3 |
|
|