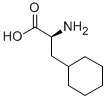
| Identification | More | [Name]
L-Cyclohexylalanine | [CAS]
27527-05-5 | [Synonyms]
2-AMINO-3-CYCLOHEXYLPROPANOIC ACID
3-CYCLOHEXYL-L-ALANINE
3-CYCLOHEXYL-L-ALANINE HYDROCHLORIDE
3-CYCLOHEXYL-L-ALANINE HYDROCHLORIDE SALT
BETA-CYCLOHEXYL-ALANINE HCL
BETA-CYCLOHEXYL-L-ALANINE
BETA-CYCLOHEXYL-L-ALANINE HYDROCHLORIDE
H-BETA-CYCLOHEXYL-ALA-OH HCL
H-CHA-OH
H-CHA-OH HCL
HEXAHYDRO-L-PHENYLALANINE HCL
HEXAHYDRO-L-PHENYLALANINE HYDROCHLORIDE
H-L-CHA-OH
H-PHE(HEXAHYDRO)-OH HCL
L-2-AMINO-3-CYCLOHEXYL-PROPIONIC ACID
L-2-AMINO-3-CYCLOHEXYL-PROPIONIC ACID HYDROCHLORIDE
L-2-AMINO-3-HEXAHYDROPHENYL-PROPIONIC ACID HYDROCHLORIDE
L-3-CYCLOHEXYL-ALANINE
L-3-CYCLOHEXYLALANINE HYDROCHLORIDE SALT
L-CHA | [EINECS(EC#)]
1308068-626-2 | [Molecular Formula]
C9H17NO2 | [MDL Number]
MFCD00190861 | [Molecular Weight]
171.24 | [MOL File]
27527-05-5.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powder | [Melting point ]
322 °C | [Boiling point ]
307.1±25.0 °C(Predicted) | [density ]
1.075±0.06 g/cm3(Predicted) | [storage temp. ]
Store at 0°C | [form ]
Solid | [pka]
2.33±0.10(Predicted) | [color ]
White to off-white | [InChI]
InChI=1/C9H17NO2/c10-8(9(11)12)6-7-4-2-1-3-5-7/h7-8H,1-6,10H2,(H,11,12)/t8-/s3 | [InChIKey]
ORQXBVXKBGUSBA-SBYBRXNCNA-N | [SMILES]
C1(CCCCC1)C[C@H](N)C(=O)O |&1:7,r| | [CAS DataBase Reference]
27527-05-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [F ]
10 | [Hazard Note ]
Irritant | [HS Code ]
2922498590 |
| Questions And Answer | Back Directory | [Description]
L-Cyclohexylalanine is a kind of amino acid analog. It has some pharmacological effects. Study has shown that some of its derivatives exhibit potent bactericidal activity against drug-resistant gram-positive pathogens. Its derivatives can also act as dipeptidyl peptidase-IV inhibitor, which can be used for the treatment or prevention of diabetes.
| [References]
http://nizetlab.ucsd.edu/Publications/CombinatorialLibrary.pdf
Duffy, Joseph L, et al. "Cyclohexylalanine derivatives as dipeptidyl peptidase-IV inhibitors for the treatment or prevention of diabetes." CN, CN 1960990 A. 2007.
Aarstad, K, T. L. Zimmer, and S. G. Laland. "The fidelity of gramicidin S synthetase." European Journal of Biochemistry 112.2(1980):335-338.
|
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powder | [Uses]
(S)-(+)-Cyclohexylalanine | [Synthesis]
Example 2: Preparation of L-cyclohexylalanine
Dissolve or suspend 20 g (121 mmol) of L-phenylalanine in a mixture of 200 ml of deionized water, 200 ml of isopropanol and 12.2 ml (146 mmol) of 37% hydrochloric acid. 2 g of Pt/Rh catalyst (4% Pt + 1% Rh loaded on activated carbon with ~50% water content, corresponding to ~5 wt% by mass of L-phenylalanine) was added. The reaction mixture was transferred to an 11-gallon hydrogenation autoclave. It was displaced three times with nitrogen and then flushed twice with hydrogen, followed by establishing a hydrogen pressure of 8-10 bar and heating the reaction system to 50-60°C. After about 6 to 8 hours of reaction, the hydrogen uptake was completed (theoretical H2 uptake of 8.1 L). The hydrogenator was depressurized and replaced with nitrogen again three times. The reaction mixture was filtered while hot through a nut filter and the catalyst was washed with 50 ml of deionized water. The filtrate was first concentrated to a smaller volume under reduced pressure (mainly to remove isopropanol), followed by adjusting the pH of the residue to 5-6 with 50% sodium hydroxide solution. the solution was cooled to 0-10 °C, the product was collected with a nut filter, washed with 50 ml of deionized water and dried in a vacuum oven at 50-70 °C. The reaction mixture was then purified with nitrogen. Yield: 19.5 g (94.2%).
1H-NMR (500 MHz, D2O/NaOD): δ (ppm) = 0.85-1.0, 1.1-1.52, and 1.63-1.75 (all multiple peaks totaling 13H, cyclohexyl-H, and cyclohexyl-CH2), 3.3 (triple peak, 1H, α-H). |
|
|