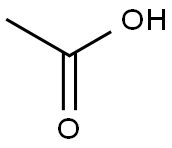
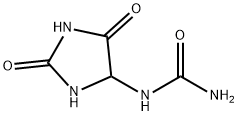
| Identification | More | [Name]
Potassium oxonate | [CAS]
2207-75-2 | [Synonyms]
4,6-DIHYDROXY-1,3,5-TRIAZINE-2-CARBOXYLIC ACID, POTASSIUM SALT
ALLANTOXANIC ACID POTASSIUM SALT
LABOTEST-BB LT00138090
OTERACIL POTASSIUM
OXONIC ACID POTASSIUM SALT
Potassium 1,4,5,6-tetrahydro-4,6-dioxo-1,3,5-triazine-2-carboxylate
POTASSIUM 4,6-DIHYDROXY-1,3,5-TRIAZINE-2-CARBOXYLATE
POTASSIUM ALLANTOXANATE
POTASSIUM OXONATE
1,4,5,6-tetrahydro-4,6-dioxo-s-triazine-2-carboxylicacid,potassiumsalt
5-azaoroticacid,potassiumsalt
kox
potassiumazaorotate
oxonic acid potassium
OXONIC ACID POTASSIUM SALT 97.5%
Oxonic acid, potassium salt, 97.50%
POTASSIUMOXONATE/OTERACILPOTASSIUM
Oteracil potassium(OXO)
4,6-Dihydroxy-1,3,5-triazine-2-carboxylic acid potassium salt, Allantoxanic acid
(OXO)Oteracil potassium | [EINECS(EC#)]
218-627-5 | [Molecular Formula]
C4H2KN3O4 | [MDL Number]
MFCD00010565 | [Molecular Weight]
195.17 | [MOL File]
2207-75-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Solid | [Melting point ]
300 °C(lit.)
| [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Aqueous Base (Slightly), Water (Slightly, Heated, Sonicated) | [form ]
Powder | [color ]
White | [biological source]
rabbit | [Water Solubility ]
Soluble in hot water. | [Usage]
Antitumor effect potentiator and antitumor agent | [Detection Methods]
T | [InChI]
InChI=1S/C4H3N3O4.K/c8-2(9)1-5-3(10)7-4(11)6-1;/h(H,8,9)(H2,5,6,7,10,11);/q;+1/p-1 | [InChIKey]
IAPCTXZQXAVYNG-UHFFFAOYSA-M | [SMILES]
C1(C([O-])=O)=NC(NC(=O)N1)=O.[K+] | [CAS DataBase Reference]
2207-75-2(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [RTECS ]
RR4580000
| [HS Code ]
29349990 |
| Hazard Information | Back Directory | [Description]
Oxonic acid is a uricase inhibitor that prevents metabolism and excretion of uric acid (Item No. 16219) and induces embryotoxicity and nephrotoxicity in rats.1 It is a component of S-1, a mixture containing a prodrug of the antitumor agent 5-fluorouracil (5-FU; Item No. 14416), that suppresses the gastrointestinal toxicity of 5-FU without inhibiting its antitumor activity in rats.2 Formulations containing oxonic acid have been used to treat gastric, pancreatic, lung, head, neck, and breast carcinomas.3 | [Chemical Properties]
Off-White Solid | [Uses]
Oxonic acid potassium salt is an inhibitor of uricase. The product from Sigma has been used for the inhibition of 5-fluorouracil-induced gastrointestinal toxicity without the loss of its antitumor activity in rats. It has also been used to induce hyperuricemia in rats; as it inhibits uric acid metabolism. | [Uses]
Antitumor effect potentiator and antitumor agent | [Uses]
Oxonic acid potassium salt is an inhibitor of uricase. The product from Sigma has been used for the inhibition of 5-fluorouracil-induced gastrointestinal toxicity without the loss of its antitumor activity in rats.It has also been used to induce hyperuricemia in rats; as it inhibits uric acid metabolism. | [Definition]
ChEBI: Potassium 2,6-dihydroxytriazinecarboxylate is an organic molecular entity. | [Synthesis]
The general procedure for synthesizing potassium oxonate from glacial acetic acid and allantoin was as follows: 3.2 kg of potassium hydroxide was dissolved in 11.4 L of water, added to a 20 L reactor to dissolve, and then cooled to 0~5 °C. 46 g of potassium iodide was added, and 0.9 kg of allantoin was added in batches and stirred until completely dissolved, keeping the temperature at 0~5 °C. Subsequently, 1.8 kg of bromine was slowly added dropwise, and the dropwise time was controlled at 9 hours (the experiment was divided into the first 1-8 groups according to the difference of bromine dropwise time). After the dropwise addition was completed, stirring was continued at 0~5°C for 1 h. Then the temperature was increased to 20~25°C and the reaction was kept at this temperature for 20 h. The reaction was carried out by HPLC. After confirming that the allantoin content was less than 0.6% by HPLC, the reaction solution was cooled to 0~5°C. The pH was adjusted to 5 with acetic acid and the reaction solution was filtered. The filter cake was washed with 3 L of water at 0~5°C and then with 1 L of room temperature ethanol. Finally, potassium oxonate was allowed to precipitate from a white potassium oxide crystalline solid at room temperature. | [in vivo]
Potassium oxonate can be used in animal modeling to create hyperuricemia models. After metabolism, Potassium oxonate primarily distributes within the cells of the small intestine. In the gastrointestinal tract, Potassium oxonate is converted to cyanuric acid via two pathways: the first involves direct conversion by gut microbiota in the cecum, and the second involves conversion through xanthine oxidase, or after degradation by stomach acid into 5-aza-uracil (5-AZU), followed by conversion through aldehyde oxidase[3].
Induction of High Uric Acid[4] Background
Potassium oxonate is a selective competitive uricase inhibitor that blocks the action of hepatic uricase.
Specific Modeling Methods
Mice : Kunming ? male ? 18-22 g
Administration: 300 mg/kg ? i.p. ? once daily for 14 days
Note
(1) The animals are adapted to the environment for 1 week before the experiment. All animals have access to a standard diet and water ad libitum and are acclimated to specific pathogen-free (SPF) conditions of 22 ± 2 °C, 60 ± 5% humidity, and a fixed 12-h artificial light period.
(2) On day 15, the mice are fasted for 12 hours, anesthetized with pentobarbital sodium, and then all sacrificed.
(3) Collect mouse blood samples in 2mL EP tubes, centrifuge (3000rpm, 4°C, 10min), and then store at -80°C for biochemical determination.
(4) The kidney tissue is quickly removed, weighed and divided into two parts, one part was fixed in 4% paraformaldehyde for histopathological examination, and the other part is stored at -80°C for further biochemical determination.
Modeling Indicators Molecular changes: Increased the levels of CRE, BUN, MDA, XOD and UA.
Reduced the levels of SOD and GSH-Px in serum.
Increased the levels of IL-1β and IL-18 in both serum and kidney and the mRNA expressions of IL-1β, NLRP3, ASC and caspase1.
Histological changes: Inconspicuous boundaries between adjacent proximal tubule cells, swelling and proximal tubule necrosis.
Correlated Product(s): Hypoxanthine (HY-N0091) Opposite Product(s): Curcumin (HY-N0005) | [References]
[1] Patent: CN106083750, 2016, A. Location in patent: Paragraph 0019; 0020; 0021 |
|
|