| Identification | Back Directory | [Name]
BUTTPARK 148\07-01 | [CAS]
20780-75-0 | [Synonyms]
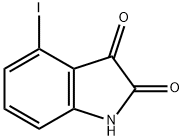
4-Iodoisatin
BUTTPARK 148\07-01
4-iodoindoline-2,3-dione
4-IODO-1H-INDOLE-2,3-DIONE
1H-Indole-2,3-dione, 4-iodo-
4-iodo-2,3-dihydro-1H-indole-2,3-dione | [Molecular Formula]
C8H4INO2 | [MDL Number]
MFCD03427300 | [MOL File]
20780-75-0.mol | [Molecular Weight]
273.03 |
| Chemical Properties | Back Directory | [Melting point ]
227-229° | [density ]
2.106±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [pka]
8.88±0.20(Predicted) | [Appearance]
Brown to orange Solid |
| Hazard Information | Back Directory | [Synthesis]
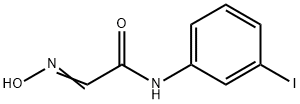
General procedure for the synthesis of 4-iodoindoline-2,3-dione from 2-(N-hydroxyimino)-N-(3-iodophenyl)acetamide: Sulfuric acid (1L) was placed in a 3L beaker on a hot plate, heated to 60°C and removed from the heat source. Compound 8a was added in batches over a period of 30 minutes under continuous stirring, controlling the reaction temperature to not exceed 65 °C. Subsequently, the reaction mixture was heated to 80 °C and maintained for 15 min, after which it was cooled to 70 °C and placed in an ice bath for further cooling. The reaction solution was slowly decanted into crushed ice (5 L) and allowed to stand for 1 h. The orange-red precipitate was collected by filtration. The product was washed with deionized water (400 mL) with stirring and filtered again to give a mixture of compound 8b with 6-iododihydroindole-2,3-dione. The crude product was dissolved in a solution of deionized water (200 mL) containing sodium hydroxide (20 g) and heated to 60 °C to dissolve it completely, followed by adjusting the pH with acetic acid to 4.9. After standing for 0.5 h and cooling to 35 °C, the precipitate of compound 8b precipitated was filtered and washed with deionized water (50 mL) to give the final product (38.37 g, 59% yield). Mass spectrometry analysis showed [M+H]+ m/z 274. | [References]
[1] Patent: WO2017/211303, 2017, A1. Location in patent: Page/Page column 40
[2] European Journal of Medicinal Chemistry, 2018, vol. 145, p. 370 - 378 |
|
|