| Identification | Back Directory | [Name]
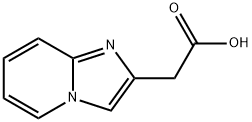
2-(1,7-diazabicyclo[4.3.0]nona-2,4,6,8-tetraen-8-yl)acetic acid | [CAS]
19741-30-1 | [Synonyms]
Nsc296220
Minodronic Impurity 2
Minodronic Acid Impurity 29
Imidazo[1,2-a]pyridin-2-acetic acid
Imidazo[1,2-a]pyridine-2-acetic acid
imidazol[1,2-A]pyridin-2-yl-acetic acid
IMIDAZO[1,2-A]PYRIDIN-2-YLACETIC ACID HYDROCHLORIDE
2-(1,7-diazabicyclo[4.3.0]nona-2,4,6,8-tetraen-8-yl)acetic acid
2-(1,7-diazabicyclo[4.3.0]nona-2,4,6,8-tetraen-8-yl)acetic acid ISO 9001:2015 REACH | [Molecular Formula]
C9H8N2O2 | [MDL Number]
MFCD03434391 | [MOL File]
19741-30-1.mol | [Molecular Weight]
176.17 |
| Chemical Properties | Back Directory | [Melting point ]
207-208 °C(Solv: ethanol (64-17-5); water (7732-18-5)) | [density ]
1.35±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [form ]
solid | [pka]
1.91±0.10(Predicted) |
| Hazard Information | Back Directory | [Synthesis]
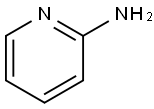
The general procedure for the synthesis of 2-(imidazo[1,2-a]pyridin-2-yl)acetic acid from 2-aminopyridine (20 g, 212 mmol) and ethyl 4-chloro-3-oxobutanoate (28.7 mL, 212 mmol) is as follows: firstly, 2-aminopyridine was added to 300 mL of ethanol, followed by the addition to a reaction vessel containing a magnetic stir bar of 4-chloro-3- ethyl oxobutyrate. The reaction mixture was stirred at 80°C for 20 hours. Upon completion of the reaction, the ethanol was evaporated and the crude product was dissolved in 100 mL of 0.5 N hydrochloric acid. The aqueous phase was extracted three times with 50 mL of dichloromethane to remove organic impurities. The product was retained in the aqueous phase and 37 g of crude product was obtained by concentration under reduced pressure. The crude product was purified by silica gel chromatography using a dichloromethane/methanol gradient elution (yield: 8.3 g, 19% yield). The resulting ester was dissolved in 200 mL of methanol and 7 mL of 10 M sodium hydroxide solution was added. The reaction mixture was stirred at room temperature for 12 h until the ester was completely saponified. After evaporation of methanol, the aqueous phase was acidified to pH=7. The precipitated product was filtered and washed with ether (yield: 7.6 g, 99%).LCMS (Method 1): retention time (Rt) = 0.435 min; HRMS (ESIpos): m/z [M + H]+ C9H8N2O2 Calculated value 177.0659, measured value 177.0660. | [References]
[1] Patent: EP3275885, 2018, A1. Location in patent: Paragraph 0118; 0119 |
|
|