| Hazard Information | Back Directory | [Synthesis]
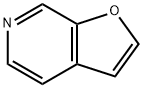
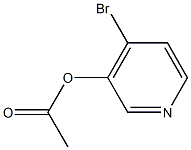
General procedure for the synthesis of furan[2,3-c]pyridines from [4-bromopyridin-3-yl] acetate: a solution of tetrahydrofuran (1.6 L) was prepared to a solution containing [4-bromopyridin-3-yl] acetate (16.0 g, 74.06 mmol, 1.0 eq.), trimethylmethylsilylacetylene (9.43 g, 96.78 mmol, 1.3 eq.) and triethylamine (112.2 g, 1110.9 mmol, 15 equiv) in a solution of tetrahydrofuran (1.6 L). The reaction mixture was degassed for 10 min and carried out under argon atmosphere. Subsequently, [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloride (1.6 g, 2.22 mmol, 0.03 eq.) and copper iodide (0.843 g, 4.44 mmol, 0.06 eq.) were added, and the reaction mixture was again degassed for 10 min and kept under argon atmosphere. The reaction mixture was stirred at room temperature for 24 hours. After completion of the reaction, the reaction mixture was filtered and the residue was concentrated under vacuum. The residue was diluted with methanol (2.2 L) and potassium fluoride was added to the reaction mixture. The reaction mixture was stirred at room temperature for 48 hours. Upon completion of the reaction, the reaction mixture was filtered through a bed of diatomaceous earth and washed with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was further purified by column chromatography using a hexane solution of 10% ethyl acetate as eluent to give pure furan[2,3-c]pyridine (3.82 g, 43.30%). Mass spectrum (electrospray ionization): m/z 120.12 [M + H]+. | [References]
[1] Patent: WO2018/71794, 2018, A1. Location in patent: Paragraph 00915; 00917 |
|
| Company Name: |
Synchem OHG
|
| Tel: |
+49 5662 408730 |
| Website: |
www.synchem.de |
|