| Identification | More | [Name]
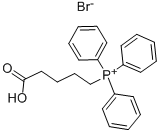
(4-Carboxybutyl)triphenylphosphonium bromide | [CAS]
17814-85-6 | [Synonyms]
(4-CARBOXYBUTYL)TRIPHENYLPHOSPHONIUM BROMIDE
CBT
(4-carboxybutyl)triphenyl-phosphoniubromide
Phosphonium, (4-carboxybutyl)triphenyl-, bromide
4-CARBOXYBUTYLTRIPHENYLPHOSPHONIUM BROMIDE 98%
(4-Carboxybutyl)triphenylphosphonium bromide, 98 %
(5-Hydroxy-5-oxopentyl)-triphenylphosphanium bromide | [EINECS(EC#)]
241-782-5 | [Molecular Formula]
C23H24BrO2P | [MDL Number]
MFCD00011906 | [Molecular Weight]
443.31 | [MOL File]
17814-85-6.mol |
| Chemical Properties | Back Directory | [Appearance]
WHITE TO ALMOST WHITE CRYSTALLINE POWDER | [Melting point ]
204-207 °C(lit.)
| [Boiling point ]
324.7-327.4℃ at 101.93-102.15kPa | [bulk density]
360-400kg/m3 | [density ]
1.371 at 20℃ | [vapor pressure ]
0Pa at 25℃ | [Fp ]
195°(383°F) | [storage temp. ]
Store below +30°C. | [solubility ]
DMSO, Methanol, Water | [form ]
solid | [color ]
White to Off-White | [Water Solubility ]
Soluble in ethanol, methanol and soluble water. Insoluble in toluene and hexane. | [Sensitive ]
Hygroscopic | [BRN ]
3586477 | [InChIKey]
KQJPHSBFOSLICV-UHFFFAOYSA-N | [LogP]
0.146 | [CAS DataBase Reference]
17814-85-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
3278 | [WGK Germany ]
3
| [HS Code ]
29310095 |
| Hazard Information | Back Directory | [Chemical Properties]
WHITE TO ALMOST WHITE CRYSTALLINE POWDER | [Uses]
suzuki reaction | [reaction suitability]
reaction type: C-C Bond Formation | [Synthesis]
General method: 5-Bromovaleric acid (1 eq.) and triphenylphosphine (1 eq.) were dissolved in 300 mL of toluene and the reaction was refluxed under argon protection for 48 hours. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently concentrated under reduced pressure. The target product 4-carboxybutyltriphenylphosphonium bromide was obtained by crystallization and purification of the residue from a suitable solvent. | [References]
[1] Journal of Organic Chemistry, 1999, vol. 64, # 9, p. 3196 - 3206
[2] Canadian Journal of Chemistry, 2003, vol. 81, # 6, p. 697 - 704
[3] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 1, p. 567 - 579
[4] Journal of Organic Chemistry, 1987, vol. 52, # 20, p. 4449 - 4453
[5] Journal of Medicinal Chemistry, 2017, vol. 60, # 14, p. 6353 - 6363 |
|
|