| Identification | More | [Name]
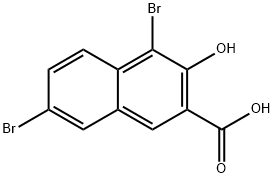
1,6-DIBROMO-2-HYDROXYNAPHTHALENE-3-CARBOXYLIC ACID | [CAS]
1779-10-8 | [Synonyms]
1,6-DIBROMO-2-HYDROXYNAPHTHALENE-3-CARBOXYLIC ACID
1,6-DIBROMO-2-NAPHTHOL-3-CARBOXYLIC ACID
4,7-DIBROMO-3-HYDROXY-2-NAPHTHOIC ACID
LABOTEST-BB LT00455402
4,7-dibromo-3-hydroxy-2-naphthalenecarboxylicaci
1,6-DIBROMO-2-HYDROXYNAPHTHALENE-3-CARB&
1,6-Dibromo-2-hydroxynaphthalene-3-carboxylic acid, 98+%
1,6-Dibromo-2-hydroxynaphthalene-3-carboxylic acid 97% | [EINECS(EC#)]
217-214-7 | [Molecular Formula]
C11H6Br2O3 | [MDL Number]
MFCD00046367 | [Molecular Weight]
345.97 | [MOL File]
1779-10-8.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36:Irritating to the eyes. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [TSCA ]
T | [HazardClass ]
IRRITANT | [HS Code ]
29181990 |
| Hazard Information | Back Directory | [Chemical Properties]
Light yellow solid | [Synthesis]
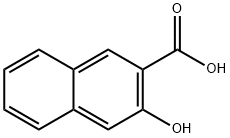
In a 1 L three-necked flask, 3-hydroxy-2-naphthalenecarboxylic acid (50 g, 0.27 mol) was dissolved in 600 mL of glacial acetic acid and stirred until completely dissolved. Another 100 mL of glacial acetic acid was taken to dilute the bromine (34 mL, 0.67 mol), and the diluted bromine solution was slowly added dropwise to the reaction solution, controlling the reaction temperature between 20-30 °C. After the dropwise addition was completed, the reaction system was heated up to 120 °C, and the reaction was refluxed for 3 hours. After the reaction was completed, the heating was stopped and the reaction solution was allowed to cool naturally to room temperature. The cooled reaction solution was slowly poured into 3000 mL of ice water, and a large amount of yellow solid was precipitated. The solid product was collected by filtration, and the filter cake was washed with water and dried in an oven to give 84 g of intermediate 2 in 91% yield. | [References]
[1] Patent: CN106866433, 2017, A. Location in patent: Paragraph 0075; 0076; 0077
[2] Patent: EP2573067, 2013, A1. Location in patent: Paragraph 0051; 0052
[3] Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 19, p. 791
[4] Journal of Medicinal Chemistry, 1990, vol. 33, # 1, p. 171 - 178
[5] Journal of Medicinal Chemistry, 2008, vol. 51, # 15, p. 4685 - 4698 |
|
|