| Identification | More | [Name]
3,4-Diaminofurazan | [CAS]
17220-38-1 | [Synonyms]
3,4-Diamino-1,2,5-oxadiazole
3,4-DIAMINOFURAZAN
3,4-DIAMINOFURAZANE
4-AMINO-1,2,5-OXADIAZOL-3-YLAMINE
AKOS BB-9341
FURAZAN-3,4-DIAMINE
Furazandiamine
TIMTEC-BB SBB000032
VITAS-BB TBB000397
3,4-DIAMINOFURAZAN/FURAZANDIAMINE
3,4-DIAMINOFURAZAN 99% | [Molecular Formula]
C2H4N4O | [MDL Number]
MFCD00138084 | [Molecular Weight]
100.08 | [MOL File]
17220-38-1.mol |
| Chemical Properties | Back Directory | [Appearance]
white to slightly beige crystalline powder | [Melting point ]
178-183 °C
| [Boiling point ]
304.0±45.0 °C(Predicted) | [density ]
1.582±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [form ]
Crystalline Powder | [pka]
1.31±0.47(Predicted) | [color ]
White to slightly beige | [InChI]
InChI=1S/C2H4N4O/c3-1-2(4)6-7-5-1/h(H2,3,5)(H2,4,6) | [InChIKey]
JHJVSUCUNFXIHN-UHFFFAOYSA-N | [SMILES]
O1N=C(N)C(N)=N1 | [CAS DataBase Reference]
17220-38-1(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3 | [HazardClass ]
IRRITANT | [HS Code ]
29349990 |
| Hazard Information | Back Directory | [Description]
3,4-Diaminofurazan (DAF) is a simple furanocyclic compound. Since DAF molecule has two highly reactive neighbouring amino groups, it is considered to be the basic structure for the preparation of other complex energetic compounds by oxidation, acylation and azide reactions. Energetic compounds based on DAF include: aminonitrofurazan (ANF), 3,4-dinitrofurazan (DNF), 4,4-diamino-3,3-diazofurazan (DAAF) and 4,4-diamino-3,3-diazofurazan (DAAzF). | [Chemical Properties]
white to slightly beige crystalline powder | [Uses]
3,4-Diaminofurazan is a building block used to prepare magnesium and zinc alkynylated salicylaldehyde-diamine Schiff base complexes. 3,4-Diaminofurazan is also used to prepare aryl(hydroxy)oxadiazolopyrazines via oxidation of acetophenones followed by heterocyclization with antibacterial-activities. | [Synthesis]
The general procedure for the synthesis of 3,4-diaminofurazan from diaminoglyoxime is as follows:
Example 1: Synthesis of 3,4-diaminofurazan.
In a 500 mL round-bottomed flask equipped with a mechanical stirrer and a thermometer, ethylene glycol (150 mL) was heated to 120 °C. To this hot solution, diaminoglyoxime (50 g, 0.42 mol) and potassium hydroxide (24 g, 0.42 mol) were added sequentially. The reaction mixture was warmed to 170 °C and maintained at this temperature for 1 hour. Upon completion of the reaction, the clarified reaction solution was cooled to room temperature and subsequently slowly poured into a mixture consisting of ice (500 g) and water (100 mL). The mixture was stirred vigorously for 5 minutes until 3,4-diaminofurazan precipitated as solid crystals. The precipitate was collected by filtration, washed with cold water (20 mL) and subsequently dried in air overnight to give 22 g of an off-white solid product (52% yield) with a melting point of 179-181 °C (literature value: Gunasekaran, A. et al., 179-180 °C). The 1H NMR (DMSO-d6) data of the product were as follows: δ 5.81 (s) ppm. | [structure and hydrogen bonding]
The unit cell parameter for monoclinic crystals of 3,4-Diaminofurazan at 20℃: a = 3.6035(3), b = 11.141(2), c = 10.329(1)Å, β = 94.80(1) , V = 413.2(1)Å3, Z = 4, dcalc = 1.61 g/cm 3, space group C2/c[1]
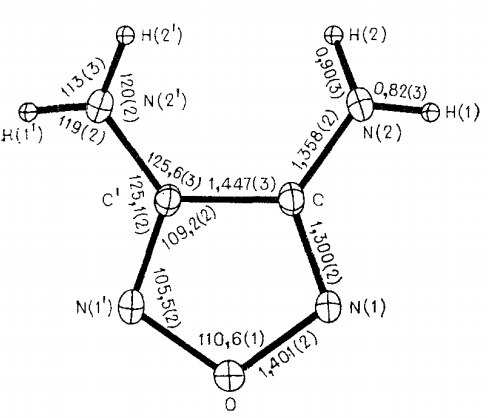 . .
| [References]
[1] A. S. Batsanov, Yu. T. Struchkov. “Crystal structure of 3,4-diaminofurazan and 3-amino-4-nitrofurazan.” Journal of Structural Chemistry 26 1 (1985): 52–56. |
|
|