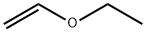| Identification | More | [Name]
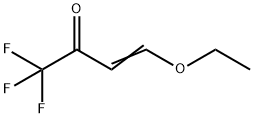
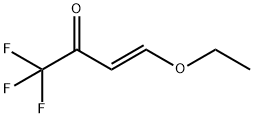
4-Ethoxy-1,1,1-trifluoro-3-buten-2-one | [CAS]
17129-06-5 | [Synonyms]
4-ETHOXY-1,1,1-TRIFLUORO-3-BUTEN-2-ONE
4-ETHOXY-1,1,1-TRIFLUORO-BUT-3-EN-2-ONE
AKOS B022234
ART-CHEM-BB B022234
4-ETHOXY-1,1,1-TRIFLUORO-3-BUTEN-2-ONE, TECH.
4-ethoxy-1,1,1-trifluoro-3-buten-2-on
(3E)-4-Ethoxy-1,1,1-trifluorobut-3-en-2-one
4-Ethoxy-1,1,1-trifluoro-3-buten-2-one, 94%, stab.
(E)-4-ethoxy-1,1,1-trifluorobut-3-en-2-one | [EINECS(EC#)]
629-004-6 | [Molecular Formula]
C6H7F3O2 | [MDL Number]
MFCD00192131 | [Molecular Weight]
168.11 | [MOL File]
17129-06-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
-30℃ | [Boiling point ]
51-53 °C/12 mmHg (lit.) | [density ]
1.18 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.406(lit.)
| [Fp ]
125 °F
| [storage temp. ]
2-8°C | [solubility ]
soluble in Chloroform, Methanol | [form ]
Liquid | [color ]
Colorless to yellow | [Sensitive ]
Moisture Sensitive | [InChI]
InChI=1S/C6H7F3O2/c1-2-11-4-3-5(10)6(7,8)9/h3-4H,2H2,1H3 | [InChIKey]
YKYIFUROKBDHCY-ONEGZZNKSA-N | [SMILES]
C(F)(F)(F)C(=O)C=COCC | [LogP]
1.414 (est) | [CAS DataBase Reference]
17129-06-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,F | [Risk Statements ]
R10:Flammable.
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S36:Wear suitable protective clothing . | [RIDADR ]
UN 1224 3/PG 3
| [WGK Germany ]
3
| [Hazard Note ]
Flammable/Harmful | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29147000 |
| Hazard Information | Back Directory | [Chemical Properties]
light yellow liquid | [Uses]
4-Ethoxy-1,1,1-trifluoro-3-buten-2-one may be used in the synthesis of 4-dialkylamino-1,1,1-trifluorobut-3-ene-2-ones. It may be used for the synthesis of β-alkyl- or dialkylamino substituted enones bearing a CF3 group. | [General Description]
4-Ethoxy-1,1,1-trifluoro-3-buten-2-one reacts with phenylmagnesium bromide to afford ethoxy group substitution products, while it reacts with organozinc compounds to yield products arising from 1,2-addition to the carbonyl group. 4-Ethoxy-1,1,1-trifluoro-3-buten-2-one on heating with triethyl phosphite affords [4+2] cycloaddition product, 2,2,2-triethoxy 2,3-dihydro-3-ethoxy-5-trifluoromethyl-1,2λ5-oxaphospholene, which on hydrolysis affords a 2-oxo-2-hydroxy-2, 3-dihydro-3-hydroxy 5-trifluoromethyl-1,2λ5-oxaphospholen. It reacts with amino to give the N-protected amino acids, which is useful for the peptide synthesis. | [Synthesis]
Example 1 Preparation of 4-ethoxy-1,1,1-trifluoro-3-buten-2-one
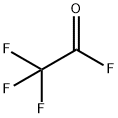
Ethyl vinyl ether (60 g) and triethylamine (28 g) were placed in an autoclave. The reaction vessel was cooled to 10°C to 15°C with continuous stirring. Trifluoroacetyl fluoride (99 g) was added in batches through an impregnation line of stainless steel cylinders at a temperature of 30°C to 40°C and a pressure of 5 to 10 kg/cm2 . After the addition was completed, the reaction mixture was continued to be stirred for 30 min until the reactor pressure was reduced to 0.25 to 0.5 kg/cm2. Excess trifluoroacetyl fluoride was removed by distillation under reduced pressure, followed by washing the reaction mixture with water. The organic layer was separated and concentrated by decompression to give the title product 4-ethoxy-1,1,1-trifluoro-3-buten-2-one. Yield: 86.2%; purity: 98.2%. | [References]
[1] Patent: WO2015/11728, 2015, A1. Location in patent: Page/Page column 7 |
|
|