| Identification | Back Directory | [Name]
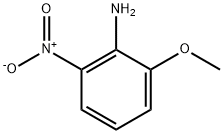
2-METHOXY-6-NITRO-PHENYLAMINE | [CAS]
16554-45-3 | [Synonyms]
Einecs 240-617-4
6-nitro-o-anisidine
2-amino-3-nitroanisole
2-METHOXY-6-NITROANILINE
2-Nitro-6-(methoxy)aniline
2-methoxy-6-nitrobenzenamine
2-METHOXY-6-NITRO-PHENYLAMINE
2-Methoxy-6-Nitrophenylamine95%
BenzenaMine, 2-Methoxy-6-nitro-
2-Methoxy-6-NitrophenylaMine 95%
2-AMino-3-nitroanisole[2-Methoxy-6-nitroaniline]
2-METHOXY-6-NITRO-PHENYLAMINE ISO 9001:2015 REACH | [EINECS(EC#)]
240-617-4 | [Molecular Formula]
C7H8N2O3 | [MDL Number]
MFCD01930197 | [MOL File]
16554-45-3.mol | [Molecular Weight]
168.15 |
| Chemical Properties | Back Directory | [Melting point ]
73-78℃ | [Boiling point ]
322.1±22.0 °C(Predicted) | [density ]
1.318±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
solid | [pka]
-0.35±0.25(Predicted) | [color ]
Light yellow to brown |
| Hazard Information | Back Directory | [Uses]
2-Methoxy-6-nitroaniline is useful for preparing pyrimidine derivatives as FGFR4 inhibitors. | [Synthesis]
Step 1: Synthesis of 2-methoxy-6-nitroaniline; A mixture of 2-amino-3-nitrophenol (11.66 g, 76 mmol) and potassium carbonate (K2CO3, 12.55 g, 91 mmol) was stirred in N,N-dimethylformamide (DMF, 100 mL) for 1 hour. Subsequently, a solution of iodomethane (5.68 mL, 91 mmol) in DMF (10 mL) was added dropwise and the reaction mixture was continued to be stirred for 14 hours. After completion of the reaction, the reaction mixture was diluted with distilled water (H2O) and extracted with ethyl acetate (EtOAc, 2 × 100 mL). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated to give a dark solid. The crude product was purified by recrystallization from hexane to give 2-amino-3-nitroanisole as an orange solid (11.6 g, 91% yield). Liquid chromatography-mass spectrometry (LCMS, ES+) showed m/z 169.0 ([M + H]+). | [References]
[1] Patent: WO2012/40040, 2012, A1. Location in patent: Page/Page column 47-48
[2] Journal of Medicinal Chemistry, 2006, vol. 49, # 17, p. 5352 - 5362
[3] Patent: US2012/88767, 2012, A1. Location in patent: Page/Page column 16; 22
[4] Journal of Asian Natural Products Research, 2011, vol. 13, # 4, p. 330 - 340
[5] European Journal of Medicinal Chemistry, 2013, vol. 68, p. 222 - 232 |
|
|