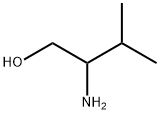
| Identification | More | [Name]
DL-2-AMINO-3-METHYL-1-BUTANOL | [CAS]
16369-05-4 | [Synonyms]
2-AMINO-3-METHYL-1-BUTANOL
DL-2-AMINO-3-METHYL-1-BUTANOL
DL-VALINOL
rac-(R*)-2-Amino-3-methylbutane-1-ol
rac-(R*)-3-Methyl-2-amino-1-butanol | [EINECS(EC#)]
240-425-0 | [Molecular Formula]
C5H13NO | [MDL Number]
MFCD00004730 | [Molecular Weight]
103.16 | [MOL File]
16369-05-4.mol |
| Chemical Properties | Back Directory | [Appearance]
CLEAR YELLOW LIQUID | [Melting point ]
31-32℃ | [Boiling point ]
75-77 °C8 mm Hg(lit.) | [density ]
0.936 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.4543(lit.)
| [Fp ]
194 °F
| [storage temp. ]
Refrigerator (+4°C) | [Water Solubility ]
Soluble in water | [form ]
Liquid | [pka]
12.82±0.10(Predicted) | [color ]
Clear yellow | [Optical Rotation]
0.3825°(C=1g/100ml ETOH) | [InChI]
InChI=1S/C5H13NO/c1-4(2)5(6)3-7/h4-5,7H,3,6H2,1-2H3 | [InChIKey]
NWYYWIJOWOLJNR-UHFFFAOYSA-N | [SMILES]
C(O)C(N)C(C)C | [CAS DataBase Reference]
16369-05-4(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves . | [RIDADR ]
2735 | [WGK Germany ]
3
| [HS Code ]
29221990 |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR YELLOW LIQUID | [Uses]
2-Amino-3-methyl-1-butanol was used in the synthesis of 1-allyl-2-pyrroleimines. It was also used as chiral nucleophile in the total synthesis of benanomicin-pradimicin antibiotics. It was used as homochiral guest compound during synthesis of crown ethers containing two chiral subunits. | [Definition]
ChEBI:2-Amino-3-methyl-1-butanol is an amino alcohol. | [Synthesis]
Example 2: To a dry 12 liter glass reaction vessel (with equipment as described in Example 1) was added 900 grams (7.7 moles) of DL-valine and 2.5 liters of tetrahydrofuran. 1.05 liters (8.5 moles) of boron trifluoride diethyl ether compound and 0.85 liters (8.5 moles) of borane-dimethyl sulfide complex were added sequentially according to the method of Example 1, followed by refluxing. The addition process lasted for 8 hours and heating was continued for 3 hours after the addition was completed. The reaction mixture was quenched with 0.75 liters of tetrahydrofuran/water mixture and subsequently hydrolyzed with 24.6 moles of aqueous sodium hydroxide. The product was separated according to the method described in Example 1, resulting in 494 g (62% yield) of 2-amino-3-methyl-1-butanol having a boiling point of 78°-79°C (8 mm), refractive index n20D 1.4543, and a purity of 97% by gas chromatography (glc) analysis. | [References]
[1] Patent: US3935280, 1976, A |
|
|