| Identification | Back Directory | [Name]
HYDROXYMETHYL EDOT | [CAS]
146796-02-3 | [Synonyms]
EDOT-OH
EDT-methanol
HYDROXYMETHYL EDOT
HydroxyMethyl EDOT 95%
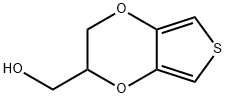
Thieno[3,4-b]-1,4-dioxin-2-methanol
2H,3H-THIENO[3,4-B][1,4]DIOXIN-2-YLMETHANOL
2,3-dihydro-Thieno[3,4-b]-1,4-dioxin-2-methanol
2,3-Dihydrothieno[3,4-b][1,4]dioxin-3-ylmethanol
Thieno[3,4-b]-1,4-dioxin-2-methanol, 2,3-dihydro
(2,3-Dihydrothieno[3,4-b][1,4]dioxin-2-yl)methanol
(2,3-Dihydrothieno[3,4-b][1,4]dioxin-2-yl)methanol, EDT-methanol, Thieno[3,4-b]-1,4-dioxin-2-methanol | [Molecular Formula]
C7H8O3S | [MDL Number]
MFCD11045357 | [MOL File]
146796-02-3.mol | [Molecular Weight]
172.203 |
| Chemical Properties | Back Directory | [Melting point ]
42-46 °C | [Boiling point ]
120°C/0.05mmHg(lit.) | [density ]
1.362±0.06 g/cm3(Predicted) | [Fp ]
110 °C | [storage temp. ]
2-8°C | [form ]
powder to crystal | [pka]
14.10±0.10(Predicted) | [color ]
White to Brown | [InChI]
InChI=1S/C7H8O3S/c8-1-5-2-9-6-3-11-4-7(6)10-5/h3-5,8H,1-2H2 | [InChIKey]
YFCHAINVYLQVBG-UHFFFAOYSA-N | [SMILES]
O1C(CO)COC2=CSC=C12 |
| Hazard Information | Back Directory | [Uses]
EDOT derivative useful in preparation of functional electroactive polymers | [General Description]
Hydroxymethyl EDOT (EDT-methanol) is a conjugated polymer that is used as a precursor of ethylenedioxythiophene (EDOT). The hydroxymethyl groups in the EDOT monomers enhance the electro-polymerization in an aqueous solution to form an electro-active hydrophilic polymer. | [Synthesis]
The general procedure for the synthesis of thieno[3,4-B]-1,4-dioxin-2-methanol from the compound (CAS:146796-14-7) was as follows: under nitrogen protection, 135.9 g of 2-hydroxymethyl-2,3-dihydro-thieno[3,4-b][1,4]dioxin-5,7-dicarboxylic acid, 8.3 g (0.10 mol) of copper(II) oxide and 2700.0 g of N,N-dimethylformamide were added to a 5 L four-neck detachable flask equipped with a stirrer, thermometer and condenser tube. The reaction was continuously heated and stirred at 130-132 °C for 8 hours. Upon completion of the reaction, the disappearance of the peak for 2-hydroxymethyl-2,3-dihydro-thieno[3,4-b][1,4]dioxin-5,7-dicarboxylic acid was confirmed by liquid chromatography analysis, and gas chromatography analysis showed the appearance of the main peak. The reaction solution was concentrated and cooled to below 25 ℃, dissolved in 1100 mL of toluene, filtered and washed sequentially with 1% aqueous hydrochloric acid and saturated saline, and concentrated again. The concentrate was purified by column chromatography and 76.0 g of light yellow solid was obtained after drying. The compound was analyzed by 1H-NMR, 13C-NMR and GC-MS, and the gas chromatographic determination showed that the purity of the main component of the obtained compound was 99.4%, which was confirmed to be 2-hydroxymethyl-2,3-dihydro-thieno[3,4-b][1,4]dioxin. | [References]
[1] Patent: JP2015/182973, 2015, A. Location in patent: Paragraph 0113; 0115; 0116
[2] Patent: JP2015/67606, 2015, A. Location in patent: Paragraph 0072
[3] Patent: CN108329329, 2018, A. Location in patent: Paragraph 0072; 0029; 0033; 0036; 0040; 0043; 0047
[4] Journal de Chimie Physique et de Physico-Chimie Biologique, 1998, vol. 95, # 6, p. 1258 - 1261
[5] Patent: WO2006/73968, 2006, A2. Location in patent: Page/Page column 23 |
|
|





![Thieno[3,4-b]-1,4-dioxin-5,7-dicarboxylic acid, 2,3-dihydro-2-(hydroxymethyl)-](/CAS/20200611/GIF/146796-14-7.gif)
