| Identification | More | [Name]
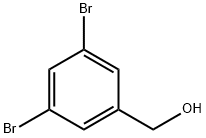
3,5-DIBROMOBENZYL ALCOHOL | [CAS]
145691-59-4 | [Synonyms]
3,5-DIBROMOBENZYL ALCOHOL
RARECHEM AL BD 0770 | [Molecular Formula]
C7H6Br2O | [MDL Number]
MFCD01632143 | [Molecular Weight]
265.93 | [MOL File]
145691-59-4.mol |
| Chemical Properties | Back Directory | [Melting point ]
107-108°C | [Boiling point ]
328℃ | [density ]
1.960 | [Fp ]
152℃ | [storage temp. ]
Sealed in dry,Room Temperature | [pka]
13.98±0.10(Predicted) | [Appearance]
Light brown to brown Solid | [Water Solubility ]
Slightly soluble in water. | [InChI]
InChI=1S/C7H6Br2O/c8-6-1-5(4-10)2-7(9)3-6/h1-3,10H,4H2 | [InChIKey]
ZQNSHKZQTZSNTB-UHFFFAOYSA-N | [SMILES]
C1(CO)=CC(Br)=CC(Br)=C1 | [CAS DataBase Reference]
145691-59-4(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [HS Code ]
2906290090 |
| Hazard Information | Back Directory | [Uses]
It is used in the synthesis of substituted benzylamines. | [Synthesis]
Step 1: To a stirred solution of 3,5-dibromobenzoic acid (2 g, 7.168 mmol) in tetrahydrofuran (20 mL) was slowly added borane dimethyl sulfide (3.4 mL, 35.842 mmol, 5 eq.) at 0 °C. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the mixture was cooled to 0 °C and the reaction was carefully quenched with methanol (appropriate amount). Subsequently, the reaction mixture was concentrated under reduced pressure to afford (3,5-dibromophenyl)methanol as an off-white solid (1.8 g, 94% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 4.47 (d, J = 6Hz, 2H), 5.39 (t, J = 6Hz, 1H), 7.49 (s, 2H), 7.65 (s, 1H). | [References]
[1] Patent: WO2017/46739, 2017, A1. Location in patent: Page/Page column 60
[2] Patent: JP2005/120047, 2005, A. Location in patent: Page/Page column 165
[3] Synlett, 2002, # 2, p. 251 - 254
[4] European Journal of Medicinal Chemistry, 2011, vol. 46, # 9, p. 4227 - 4237 |
|
|