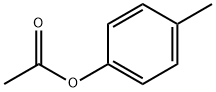
| Identification | More | [Name]
1-(2-Hydroxy-5-methylphenyl)ethanone | [CAS]
1450-72-2 | [Synonyms]
1-(2-HYDROXY-5-METHYLPHENYL)-1-ETHANONE
1-(2-HYDROXY-5-METHYLPHENYL)ETHAN-1-ONE
1-(2-HYDROXY-5-METHYLPHENYL)ETHANONE
2-ACETYL-4-METHYLPHENOL
2'-HYDROXY-5'-METHYLACETOPHENONE
2-HYDROXY-5-METHYLACETOPHENONE
ASISCHEM D38759
AURORA 16683
OTAVA-BB BB7013941401
TIMTEC-BB SBB005399
1-(2-hydroxy-5-methylphenyl)-ethanon
1-Hydroxy-2-acetyl-4-methylbenzene
2’-Hydroxy-5’-methylacetophenon
5’-Methyl-2’-hydroxyacetophenone
Acetophenone, 2'-hydroxy-5'-methyl-
Acetophenone,2’-hydroxy-5’-methyl-
Ethanone,1-(2-hydroxy-5-methylphenyl)-
o-Acetyl-p-cresol
2-Hydroxy-5-methylacetophenone ,98% | [EINECS(EC#)]
215-915-2 | [Molecular Formula]
C9H10O2 | [MDL Number]
MFCD00002380 | [Molecular Weight]
150.17 | [MOL File]
1450-72-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29147000 |
| Hazard Information | Back Directory | [Chemical Properties]
yellow crystalline powder | [Uses]
2'-Hydroxy-5'-methylacetophenone is used to produce 2-ethyl-4-methyl-phenol. It is used as an intermediate in organic synthesis. | [Definition]
ChEBI: 2'-Hydroxy-5'-methylacetophenone is an aromatic ketone. | [Preparation]
Obtained by treatment of methyl 4-hydroxy-6-methyl-coumarin-3-carboxylate with potassium hydroxide at 200° (82%). | [Synthesis]
The general procedure for the synthesis of 2-hydroxy-5-methylacetophenone from p-cresyl acetate was as follows: p-tolyl acetate (10 g, 0.067 mol) and aluminum chloride (10.7 g, 0.08 mol) were added to a 500-mL round-bottomed flask. The reaction mixture was heated in an oil bath at 140-150°C for 5-6 hours. The reaction process was monitored by thin layer chromatography (TLC) using ethyl acetate:hexane as the unfolding agent. After completion of the reaction, the reaction mixture was quenched with crushed ice and the resulting solid product was extracted with ethyl acetate (2 x 50 mL). The organic phase was washed with brine solution (2 x 15 mL) and dried with anhydrous sodium sulfate. Subsequently, the solvent was removed by distillation under reduced pressure to give the crude product. The crude product was purified by aqueous ethanol recrystallization to afford the target compound 2-hydroxy-5-methylacetophenone in 92% yield with a melting point of 45-48°C. | [References]
[1] Journal of Organic Chemistry, 1981, vol. 46, p. 4971 - 4975
[2] Journal of Chemical Research, Miniprint, 2003, # 12, p. 1258 - 1270
[3] Journal of Chemical Research, Synopses, 1999, # 9, p. 574 - 575
[4] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1996, vol. 35, # 7, p. 734 - 736
[5] Journal of Chemical Research, Miniprint, 1998, # 10, p. 2678 - 2695 |
|
|