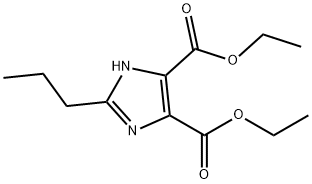
| Identification | More | [Name]
Diethyl 2-propylImidazoledicarbonate | [CAS]
144689-94-1 | [Synonyms]
1H-IMIDAZOLE-4, 5-DICARBOXYLIC ACID,2-PROPYL-DIETHYL ESTER
2-PROPYL-1H-IMIDAZOLE-4,5-DICARBOXY ACID DIETHYL ESTER
2-PROPYL-1H-IMIDAZOLE-4, 5-DICARBOXYLIC ACID DIETHYL ESTER
DIETHYL 2-PROPYL-1H-IMIDAZOLE-4,5-DICARBOXYLATE
Diethyl 2-propylImidazoledicarbonate
2-propyl-1H-imidazole-4,5-dicarboxyaciddiethyl
2-PROPYL-1H-IMIDAZOLE-4,5-DICARBOXYL
2-Propylimidazoledicarboxylic acid diethyl ester
2-PROPYL-4,5-IMIDAZOLEDICARBOXYLIC ACID DIETHYL ESTER 98.0%
2-propyl-, diethyl ester | [EINECS(EC#)]
604-435-2 | [Molecular Formula]
C12H18N2O4 | [MDL Number]
MFCD08062365 | [Molecular Weight]
254.28 | [MOL File]
144689-94-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
86 °C | [Boiling point ]
400.9±25.0 °C(Predicted) | [density ]
1.160±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
powder to crystal | [pka]
9.25±0.10(Predicted) | [color ]
White to Yellow to Orange | [CAS DataBase Reference]
144689-94-1(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Light Yellow Solid | [Synthesis]
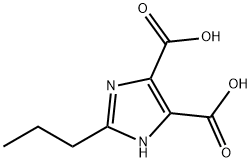
General procedure for the synthesis of diethyl 2-propyl-4,5-imidazoledicarboxylate from 2-propyl-1H-imidazole-4,5-dicarboxylic acid and ethanol: 48 mmol of 2-n-propylimidazole-4,5-dicarboxylic acid (synthesized in Example 3) was added to a four-necked flask with 152 mL of ethanol. 20.22 g (165 mmol) of thionyl chloride was added slowly dropwise at liquid temperature over 1 hour and 40 minutes. Subsequently 0.91 g (51 mmol) of water was added and the reaction solution was cooled to room temperature. The reaction mixture was heated to reflux for 5 hours (liquid temperature: 74 °C to room temperature). Upon completion of the reaction, the precipitated inorganic salt was removed by filtration. The filtrate was concentrated and partitioned for extraction by adding 50 mL of ethyl acetate and 60 mL of saturated aqueous sodium bicarbonate. After adjusting the pH of the aqueous layer to 7, 20 mL of ethyl acetate was added. The organic layers were combined, dried with anhydrous magnesium sulfate, filtered and concentrated to give the crude product of diethyl 2-propyl-4,5-imidazoledicarboxylate. To the crude product, 13.06 g of ethyl acetate and 1.07 g of ethanol were added, concentrated to a product weight of 14.5 g and crystallized. After filtration, it was dried under vacuum at 50°C to obtain diethyl 2-propyl-4,5-imidazoledicarboxylate. The first to third crystals were obtained sequentially by recrystallizing the mother liquor and repeating the recrystallization process according to the same method as in Example 1. By HPLC analysis, the yield was 9.50 g (77%) and the HPLC purity was 99.7% for the first crystal, 99.7% for the second crystal and 99.0% for the third crystal, respectively. | [References]
[1] Organic Preparations and Procedures International, 2006, vol. 38, # 4, p. 410 - 412
[2] Journal of Medicinal Chemistry, 1996, vol. 39, # 1, p. 323 - 338
[3] Patent: JP5688696, 2015, B2. Location in patent: Paragraph 0097 |
|
|