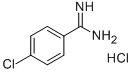
| Identification | More | [Name]
4-Chlorobenzene-1-carboximidamide hydrochloride | [CAS]
14401-51-5 | [Synonyms]
4-CHLOROBENZAMIDINE HCL
4-CHLORO-BENZAMIDINE HYDROCHLORIDE
4-CHLOROBENZENE-1-CARBOXIMIDAMIDE HYDROCHLORIDE
4-chlorobenzenecarboximidamidemonohydrochloride
4-chloro-benzenecarboximidamidmonohydrochloride
p-chloro-benzamidhydrochloride
p-chloro-benzamidinmonohydrochloride
4-Chlorobenzene-1-carboximidamideHCl
4-Chlorobenzene-1-carboximidamide hydrochloride, 95+%
4-Chlorobenzamidine hydrochloride ,95% | [EINECS(EC#)]
604-389-3 | [Molecular Formula]
C7H8Cl2N2 | [MDL Number]
MFCD00126401 | [Molecular Weight]
191.06 | [MOL File]
14401-51-5.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [RTECS ]
CV6136200 | [Hazard Note ]
Irritant | [HS Code ]
29252900 | [Toxicity]
mouse,LD50,intravenous,90mg/kg (90mg/kg),Journal of Pharmacology and Experimental Therapeutics. Vol. 84, Pg. 16, 1945. |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
4-Chlorobenzene-1-carboximidamide, HCl | [Synthesis]
The general procedure for the synthesis of 4-chlorobenzamidine hydrochloride from p-chlorobenzonitrile was as follows: a THF solution of 1 M LiHMDS (22 mmol) was added to a dry 50 mL reaction flask, followed by the addition of a 2 mL THF solution of p-chlorobenzonitrile (2.76 g, 20.0 mmol). The reaction mixture was stirred at room temperature for 4 hours. Upon completion of the reaction, 5-6N HCl (in isopropanol, 15 mL) was added. The crude reaction mixture was placed at 0°C overnight. The precipitated product was collected by filtration and washed with ether to give 3.5 g (93% yield) of 4-chlorobenzamidine hydrochloride as a white solid with a melting point of 205 °C (literature value: 243-245 °C) (E. Ragona, D. L. Nelson, M. Mares-Guis, J. Amer. Chem. Soc. EPO 1975,97,. 6844-6848).
- IR (KBr): ν = 3239, 3054, 1678, 1460, 1401, 1036, 715 cm-1.
- 1H NMR (250 MHz, [D6] DMSO): δ = 7.60-7.77 (m, 2H), 7.85-7.97 (m, 2H), 8.4 (br.s, 3H, NH).
- 13C NMR (62.9 MHz, [D6] DMSO): δ = 126.79 (Cquat), 129.36 (+), 130.57 (+), 139.1 (Cquat), 165.1 (NCN). | [References]
[1] European Journal of Organic Chemistry, 2006, # 12, p. 2753 - 2765
[2] Patent: WO2006/94604, 2006, A1. Location in patent: Page/Page column 62; 63; 72; 73
[3] Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 17, p. 5235 - 5246
[4] Journal of the American Chemical Society, 1985, vol. 107, # 9, p. 2743 - 2748
[5] Patent: US6218538, 2001, B1 |
|
|