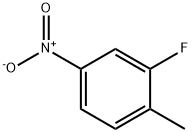
| Identification | More | [Name]
2-Fluoro-4-nitrotoluene | [CAS]
1427-07-2 | [Synonyms]
2-FLUORO-1-METHYL-4-NITROBENZENE
2-FLUORO-4-NITROTOLUENE
Toluene, 2-fluoro-4-nitro-
4-Nitro-2-Fluorotoluene/2-Fluoro-4-Nitrotoluene
4-Fluoro-4-Nitrotoluene
2-Fluoro-4-nitrotoluene 98%
2-Fluoro-4-nitrotoluene98%
2-Fluoro-4-nitrotluene
2-fluoro-4-nitrobenzene
Benzene, 2-fluoro-1-methyl-4-nitro-
1-Methyl-2-fluoro-4-nitrobenzene
1-Nitro-4-methyl-3-fluorobenzene
4-Nitro-2-fluorotoluene | [EINECS(EC#)]
215-845-2 | [Molecular Formula]
C7H6FNO2 | [MDL Number]
MFCD00007199 | [Molecular Weight]
155.13 | [MOL File]
1427-07-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powder | [Melting point ]
31-35 °C (lit.) | [Boiling point ]
65-68 °C/2 mmHg (lit.) | [density ]
1.3021 (estimate) | [Fp ]
165 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Crystalline Low Melting Solid | [color ]
Yellow to brown | [Water Solubility ]
Insoluble in water. Solubility in methanol gives very faint turbidity. | [FreezingPoint ]
32.0 to 35.0 ℃ | [BRN ]
2250156 | [InChI]
InChI=1S/C7H6FNO2/c1-5-2-3-6(9(10)11)4-7(5)8/h2-4H,1H3 | [InChIKey]
WIQISTBTOQNVCE-UHFFFAOYSA-N | [SMILES]
C1(C)=CC=C([N+]([O-])=O)C=C1F | [CAS DataBase Reference]
1427-07-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Benzene, 2-fluoro-1-methyl-4-nitro-(1427-07-2) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 1325 4.1/PG 2
| [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29049085 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powder | [Uses]
2-Fluoro-4-nitrotoluene was used in the synthesis of 2-fluoro-4-nitrobenzoic acid. | [Synthesis]
General procedure for the synthesis of 2-fluoro-4-nitrotoluene from 4-nitrotoluene: To a three-necked flask equipped with an argon inlet and a thermometer was added a solution of anhydrous acetonitrile (15 mL) of 4-nitrotoluene (10 mmol). Subsequently, bis(trifluoroethoxy)phenyl iodide(III) tetrafluoroborate (BTFE, 2.0 g, 14 mmol) was added. The reaction mixture was cooled to -35 °C under argon protection and xenon difluoride (XeF2, 2.05 g, 12.5 mmol) was added in batches. The mixture was slowly warmed to -25 °C and stirred at this temperature for 30 min, then continued to warm to 20 °C, held for 1 h and stirred for another 1 h (the reaction progress was monitored by gas chromatography). After completion of the reaction, saturated sodium bicarbonate solution was added slowly to the reaction mixture until no gas was produced. The reaction mixture was extracted with ether (3 x 20 mL), the organic phases were combined, washed with water and dried with anhydrous sodium sulfate. For the reaction products of benzene (1), toluene (3) and chlorobenzene (4), the ether extracts were molecularly distilled and fractionated, and the fractions were analyzed by gas chromatography and NMR spectroscopy. For the reaction products of bromobenzene (5), nitrobenzene (6), p-nitrotoluene (7), and p-nitroanisole (8), the ether extracts were concentrated under vacuum at less than 25 °C, and the residue was dissolved in chloroform and separated by fast column chromatography (eluent was hexane-chloroform, 3:1). The eluate was concentrated at atmospheric pressure and the residue was analyzed by NMR spectroscopy. For the reaction product of iodobenzene (15), the precipitate formed after neutralization with sodium bicarbonate solution was filtered, washed with water, and the filtrate was washed sequentially with acetonitrile (5 mL) and ether (5 mL), and the solid was dried in air to give 1.1 g of solid residue, which according to NMR spectroscopy data was a mixture of 76% iodobenzene (16) and 24% (4-iodophenyl)phenyliodonium tetrafluoroborate ( 17) comprising a mixture. The organic filtrate was evaporated and the residue was treated with chloroform to give 100 mg of compound 18 with a melting point of 149 °C (literature value: 147-149 °C). | [References]
[1] Journal of Fluorine Chemistry, 2000, vol. 102, # 1-2, p. 169 - 173
[2] Russian Chemical Bulletin, 2015, vol. 64, # 5, p. 1049 - 1052
[3] Izv. Akad. Nauk, Ser. Khim., 2015, # 5, p. 1049 - 1052,4 |
|
|