| Identification | More | [Name]
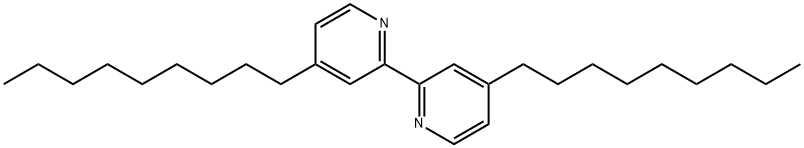
4,4'-Dinonyl-2,2'-bipyridine | [CAS]
142646-58-0 | [Synonyms]
4 4'-DINONYL-2 2'-DIPYRIDYL 97
4,4'-Dinonyl-2,2'-bipyridine
4,4-DINOYL-2,2-DIPYRIDYL
4,4'-Bis(nonyl)-2,2'-bipyridine | [Molecular Formula]
C28H44N2 | [MDL Number]
MFCD00800915 | [Molecular Weight]
408.66 | [MOL File]
142646-58-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
61-63 °C(lit.) | [Boiling point ]
522.4±45.0 °C(Predicted) | [density ]
0.933±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
powder to crystal | [pka]
5.30±0.30(Predicted) | [color ]
White to Almost white | [InChIKey]
VHJFWJXYEWHCGD-UHFFFAOYSA-N | [CAS DataBase Reference]
142646-58-0(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [HS Code ]
29333990 |
| Hazard Information | Back Directory | [Chemical Properties]
White or off-white powder | [Uses]
Used in the preparation of an artificial muscle with lamellar structure based on a nematic triblock copolymer. Also use as complex in cyclic voltammetric studies of copper complexes catalyzing atom transfer radical polymerization. | [Synthesis]
Diisopropylamine (28.0 mL, 0.160 mol, 2.66 eq.) was dissolved in anhydrous tetrahydrofuran (THF, 60 mL) under argon protection. The reaction system was cooled to 0 °C, n-butyllithium (107.0 mL, 0.160 mol, 2.66 eq.) was added slowly dropwise and stirred at this temperature for 1 hour. Subsequently, anhydrous THF (300 mL) solution of 4,4'-dimethyl-2,2'-bipyridine (11.0 g, 0.060 mol, 1 eq.) was added drop-wise, the reaction mixture took on an orange color, and stirring was continued for 3 hours. Keeping the reaction system at 0 °C, a solution of anhydrous THF (20 mL) of 1-bromooctane (28.0 mL, 0.160 mol, 2.66 equiv) was added. The reaction mixture was gradually warmed to room temperature and stirred continuously for 12 hours. Upon completion of the reaction, the reaction was first quenched with deionized water (20 mL) and then the mixture was poured into an ice-water mixture and extracted with diethyl ether. The resulting yellow oily product was purified by recrystallization from hexane and subsequently dissolved in dichloromethane (DCM), washed with dilute sodium hydroxide solution, dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure. The final product was dried under high vacuum to afford 4,4'-dinonyl-2,2'-bipyridine (11.02 g, 0.027 mol, 45% yield). The structure of the product was confirmed by 1H NMR (500 MHz, DMSO) and HRMS (ESI): 1H NMR δ 8.85 (s, 1H), 8.83 (s, 1H), 7.28 (s, 1H), 7.27 (s, 1H), 2.68 (t, J = 7.6 Hz, 4H), 1.62 (p, J = 8.2, 6.9 Hz, 4H). 1.32-1.11 (m, 24H), 0.83 (t, J = 6.5 Hz, 6H); HRMS (ESI) m/z: [M + H]+ Calculated value 409.358, measured value 409.3581. | [References]
[1] Dyes and Pigments, 2014, vol. 104, p. 24 - 33
[2] Journal of the American Chemical Society, 1998, vol. 120, # 2, p. 305 - 316 |
|
|