| Identification | More | [Name]
1-(2-N-Boc-Aminoethyl)piperazine | [CAS]
140447-78-5 | [Synonyms]
1-(2-BOC-AMINOETHYL)PIPERAZINE
1-(2-N-BOC-AMINOETHYL)PIPERAZINE
(2-PIPERAZIN-1-YL-ETHYL)-CARBAMIC ACID TERT-BUTYL ESTER
ASINEX-REAG BAS 07746391
BUTTPARK 90\06-02
TIMTEC-BB SBB011604
1-(2-N-BOC-AMINOETHYL)PIPERAZINE 97% | [Molecular Formula]
C11H23N3O2 | [MDL Number]
MFCD02683050 | [Molecular Weight]
229.32 | [MOL File]
140447-78-5.mol |
| Chemical Properties | Back Directory | [Boiling point ]
357.2±27.0 °C(Predicted) | [density ]
1.014±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Store in freezer, under -20°C | [form ]
solid | [pka]
12.74±0.46(Predicted) | [color ]
White to off-white | [InChI]
InChI=1S/C11H23N3O2/c1-11(2,3)16-10(15)13-6-9-14-7-4-12-5-8-14/h12H,4-9H2,1-3H3,(H,13,15) | [InChIKey]
VPOIPCJBJNWHSJ-UHFFFAOYSA-N | [SMILES]
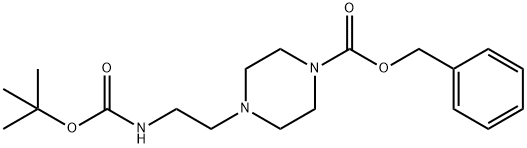
C(OC(C)(C)C)(=O)NCCN1CCNCC1 | [CAS DataBase Reference]
140447-78-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
1-[2-(Boc-amino)ethyl]piperazine is used in preparation of dihydropyrimidine derivatives as bifunctional degraders for treatment and prophylaxis of target protein-mediated diseases. | [Synthesis]
The general procedure for the synthesis of 1-(N-Boc-aminoethyl)piperazine from 1-CBZ-4-(BOC-aminoethyl)piperazine was as follows: hydrogen was vented into a degassed solution of benzyl 4-(2-(tert-butoxycarbonylamino)ethyl)piperazine-1-carboxylate (2.60 g, 7.16 mmol) containing 10% palladium carbon (500 mg) in methanol (50 ml), the reaction lasted for 2 hours. After completion of the reaction, the mixture was filtered through Celite. The filtrate was concentrated under vacuum to give a yellow colloidal product which was identified as tert-butyl 2-(1-piperazinyl)ethylcarbamate (1.60 g, 97% yield). | [References]
[1] Patent: EP1449844, 2004, A1. Location in patent: Page 34
[2] Organic and Biomolecular Chemistry, 2015, vol. 13, # 18, p. 5147 - 5157 |
|
|