| Identification | Back Directory | [Name]
GDC-0084 | [CAS]
1382979-44-3 | [Synonyms]
RG7666
RG-7666
CS-2290
GDC-0084
RG 7666)
Paxalisib
GDC-0084(RG7666)
GDC-0084 (GDC0084
Paxalisib (GDC-0084)
GDC-0084;GDC 0084;RG7666;RG-7666;RG 7666
RG7666; RG-7666; RG 7666; GDC-0084; GDC0084; GDC 0084
5-(6,6-dimethyl-4-morpholino-8,9-dihydro-6H-[1,4]oxazino[4,3-e]purin-2-yl)pyrimidin-2-amine
5-[8,9-Dihydro-6,6-dimethyl-4-(4-morpholinyl)-6H-[1,4]oxazino[4,3-e]purin-2-yl]-2-pyrimidinamine
5-[6,6-Dimethyl-4-(4-morpholinyl)-8,9-dihydro-6H-[1,4]oxazino[4,3-e]purin-2-yl]-2-pyrimidinamine
2-Pyrimidinamine, 5-[8,9-dihydro-6,6-dimethyl-4-(4-morpholinyl)-6H-[1,4]oxazino[4,3-e]purin-2-yl]- | [Molecular Formula]
C18H22N8O2 | [MDL Number]
MFCD30187522 | [MOL File]
1382979-44-3.mol | [Molecular Weight]
382.42 |
| Chemical Properties | Back Directory | [density ]
1.60±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
insoluble in H2O; insoluble in EtOH; ≥2.83 mg/mL in DMSO with ultrasonic | [form ]
Solid | [pka]
2.62±0.40(Predicted) | [color ]
White to off-white | [InChI]
InChI=1S/C18H22N8O2/c1-18(2)16-22-12-14(25-3-6-27-7-4-25)23-13(11-9-20-17(19)21-10-11)24-15(12)26(16)5-8-28-18/h9-10H,3-8H2,1-2H3,(H2,19,20,21) | [InChIKey]
LGWACEZVCMBSKW-UHFFFAOYSA-N | [SMILES]
C1(N)=NC=C(C2N=C(N3CCOCC3)C3=C(N=2)N2CCOC(C)(C)C2=N3)C=N1 |
| Hazard Information | Back Directory | [Uses]
GDC-0084 is an inhibitor of phosphoinositide 3-kinase (PI3K) and mTOR (1,2). Inhibitors of PI3K are useful compounds for developing treatments for brain tumors. | [Preparation]
Stumpf et al. at Genentech Inc. (Roche group) developed an efficient multikilogram process to synthesize the brain penetrant PI3K inhibitor, GDC?\0084, a potential drug candidate for the treatment of several brain cancers. An efficient Suzuki coupling reaction between a chloropyrimidine and an arylboronic ester using a palladium catalyst at low loading was reported. The optimized conditions were demonstrated on 6.75 kg of a chloropyrimidine intermediate, providing 7.49 kg of crude GDC?\0084 (94% yield, 99.4% HPLC purity). Using the commercially available XPhos Pd G2 catalyst, instead of the usual PdCl2(dppf)·CH2Cl2 catalyst, it was possible to reduce the catalyst loading from 2 to 0.5 mol%. A final scavenging/recrystallization combination from Si?\Thiol (a solid?\supported resin) and acetic acid/water provided the crude GDC?\0084 with the required purity and polymorphic form (off?\white solid, 6.41 kg, 83% yield, 99.7% HPLC purity).
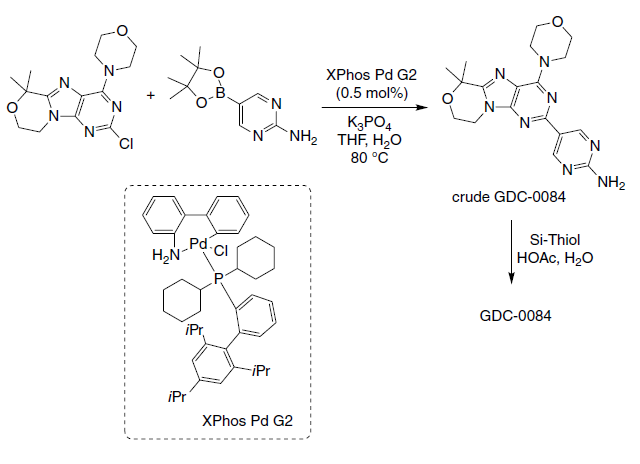
Genentech’s route to GDC?\0084 employing a pivotal Suzuki reaction. | [Biological Activity]
gdc-0084 is a potent and brain penetrant inhibitor of pi3k and mtor with ki values of 2 nm and 70 nm for pi3kα and mtor, respectively [1].glioblastoma (gbm) is the most common primary brain tumor in adults and aberrant pi3k signaling is associated with more than 80% of cases. the pi3k pathway represents a potential target for the treatment of this disease and the inhibitors would need to freely cross the blood-brain barrier (bbb) [1][2].gdc-0084 is a potent and brain penetrant inhibitor of pi3k and mtor. in vitro kinase assay, gdc-0084 exhibited ki values of 2 nm, 46 nm, 3 nm, 10 nm and 70 nm for pi3kα, pi3kβ, pi3kδ, pi3kγ and mtor, respectively. in five different gbm cell lines, gdc-0084 had antiproliferative activities with ec50 values ranging from 0.3 to 1.1 μm. gdc-0084 has excellent human metabolic stability in microsomal and hepatocyte incubations [1]. in transfected cell lines over-expressing human or mouse p-gp or bcrp, gdc-0084 was a poor substrate of these efflux transporters. in mice brain, gdc-0084 significantly lowered pakt and ps6 levels [2].in rats after a 15 mg/kg dose of gdc-0084, the total brain-to-plasma ratio was 1.9-3.3. in subcutaneous u87 glioblastoma tumor xenograft mice model, gdc-0084 significantly inhibited tumor growth in a dose-dependent way. gdc-0084 also concentration-dependently inhibited pakt [1].[1]. heffron tp1, ndubaku co1, salphati l1, et al. discovery of clinical development candidate gdc-0084, a brain penetrant inhibitor of pi3k and mtor. acs med chem lett. 2016 feb 16;7(4):351-6.[2]. salphati l, alicke b, heffron tp, et al. brain distribution and efficacy of the brain penetrant pi3k inhibitor gdc-0084 in orthotopic mouse models of human glioblastoma. drug metab dispos. 2016 dec;44(12):1881-1889. epub 2016 sep 16. | [Synthesis]
Step 3: 2-Chloro-6,6-dimethyl-4-morpholino-8,9-dihydro-6H-[1,4]oxazolo[3,4-e]purine (180 mg, 0.56 mmol) was dissolved with 2-aminopyrimidine-5-boronic acid pinacol ester (180 mg, 0.83 mmol) in 1,2-dimethoxyethane (5.1 mL) and 1.0 M cesium carbonate aqueous solution (1.7 mL). The reaction mixture was degassed for 5 min and circulated under nitrogen atmosphere. Subsequently, 1,1'-bis(diphenylphosphino)ferrocene palladium(II) chloride (54 mg, 0.067 mmol) was added, degassed again and circulated. The reaction vial was placed in a microwave reactor and radiated at 140 °C for 20 min. Upon completion of the reaction, the solid precipitate formed was collected by filtration and rinsed with excess water. The precipitate was dissolved in dichloromethane (DCM) and purified by fast column chromatography (FCC, 40 g silica gel, 0.5-4% methanol/DCM solution, slow gradient) to afford the target product, 5-(6,6-dimethyl-4-morpholino-8,9-dihydro-6H-[1,4]oxazino[4,3-e]purin-2-yl)pyrimidin-2-amine, as a tan powder (56 mg, 27% yield).MS (ESI+): m/z 383.1 (M + H+).1H NMR (400 MHz, DMSO) δ 9.09 (s, 2H), 7.00 (s, 2H), 4.23 (m, 4H), 4.13 (m, 4H), 3.79-3.68 (m, 4H), 2.50 (s, 6H). | [in vivo]
After a 25 mg/kg dose of Paxalisib (GDC-0084; Compound 16) administered orally, pAKT in normal mouse brain tissue is significantly inhibited at 1 and 6 h postdose. The potent inhibition of pAKT at both time points in this study demonstrates that Paxalisib inhibits its target behind a fully intact BBB. In addition to the pharmacodynamic effect in normal brain tissue, Paxalisib is studied in a subcutaneous U87 tumor xenograft model of glioblastoma in mice. In this study, Paxalisib achieves significant and dose-dependent tumor growth inhibition. Tumor growth inhibition is first observed at a 2.2 mg/kg dose level. Higher doses led to greater tumor growth inhibition, including tumor regressions at the 17.9 mg/kg dose level. Each of these doses is well tolerated for the duration of the study[1]. | [IC 50]
PI3Kα: 2 nM (Ki); PI3Kδ: 3 nM (Ki); PI3Kγ: 10 nM (Ki); PI3Kβ: 46 nM (Ki); mTOR: 70 nM (Ki) | [References]
[1] Organic Process Research and Development, 2016, vol. 20, # 4, p. 751 - 759
[2] Patent: WO2012/82997, 2012, A1. Location in patent: Page/Page column 91
[3] ACS Medicinal Chemistry Letters, 2016, vol. 7, # 4, p. 351 - 356 |
|
|