[Synthesis]
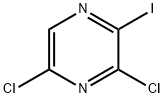
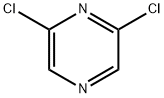
Synthesis of 3,5-dichloro-2-iodopyrazine (11a): 2,6-dichloropyrazine (9) (149 mg, 1.0 mmol) was dissolved in THF (2 mL) at 25 °C, then TMPZnCl-LiCl (1.3 M in THF, 0.85 mL, 1.1 mmol) was added. The reaction mixture was stirred at this temperature for 30 min. Subsequently, iodine (I2, 381 mg, 1.5 mmol) was dissolved in anhydrous THF (2 mL) and slowly added dropwise to the reaction system and stirring was continued for 0.5 hr. Upon completion of the reaction, the reaction was quenched with saturated aqueous NaHCO3 (10 mL) and saturated aqueous Na2S2O3 (10 mL) and extracted with ether (3 × 50 mL). The organic phases were combined, dried with anhydrous Na2SO4, filtered and concentrated under reduced pressure. Purification by fast column chromatography (CH2Cl2/n-pentane, 1:2) afforded the target product 11a (251 mg, 90%) as a colorless solid with a melting point: 101.3-103.0 °C. 1H-NMR (300 MHz, CDCl3) δ: 8.30 (s, 1H). 13C-NMR (75 MHz, CDCl3) δ: 153.1, 146.9, 142.9, 142.1. 146.9, 142.4, 115.7. m/z (%) MS (70 eV, EI): 274 (100) [M+], 147 (75), 127 (18), 86 (32), 57 (21), 44 (94). ν/cm-1 IR (ATR): 2969, 2633, 2281, 1784, 1738, 1510, 1491, 1379, 1353. 1491, 1379, 1353, 1323, 1274, 1230, 1217, 1205, 1175, 1162, 1143, 1018, 893, 843, 655, 634, 618, 611, 604. HRMS (EI) calculated value of C4HCl2IN2 (M+): 273.8561, measured value: 273.8555. |
[References]
[1] Organic Letters, 2009, vol. 11, # 8, p. 1837 - 1840
[2] Patent: US2011/288296, 2011, A1. Location in patent: Page/Page column 11
[3] Synthesis (Germany), 2016, vol. 48, # 19, p. 3155 - 3164
[4] Tetrahedron Letters, 2011, vol. 52, # 36, p. 4590 - 4594
[5] Organic Letters, 2013, vol. 15, # 9, p. 2156 - 2159 |