| Identification | Back Directory | [Name]
(1R,3S)-3-AMinocyclopentanol hydrochloride | [CAS]
1279032-31-3 | [Synonyms]
Intermediate of Bictegravir
(1R,3S)-3-aminocyclopentanolHCL
1R.3S)-3-
Aminocyclopentaro1hydroch1oj
de
1R,3S)-3-Aminocyclopentanol
hydrochloride(Bictegravir)
Cyclopentanol, 3-amino-, hydrochloride (1:1), (1R,3S)-
(1R,3S)-3-AMinocyclopentanol hydrochloride ISO 9001:2015 REACH | [Molecular Formula]
C5H12ClNO | [MDL Number]
MFCD11878010 | [MOL File]
1279032-31-3.mol | [Molecular Weight]
137.608 |
| Chemical Properties | Back Directory | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly), Water (Slightly) | [form ]
Solid | [color ]
White to Pale Yellow | [InChI]
InChI=1/C5H11NO.ClH/c6-4-1-2-5(7)3-4;/h4-5,7H,1-3,6H2;1H/t4-,5+;/s3 | [InChIKey]
SGKRJNWIEGYWGE-SRJSDOSHNA-N | [SMILES]
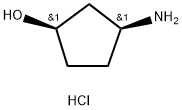
N[C@H]1CC[C@@H](O)C1.Cl |&1:1,4,r| | [CAS DataBase Reference]
1279032-31-3 |
| Hazard Information | Back Directory | [Description]
(1R,3S)-3-aminocyclopentanol hydrochloride is an alcohol derivative and can be used as a pharmaceutical intermediate. | [Uses]
(1R,3S)-3-aminocyclopentanol hydrochloride is used in the preparation of 6-aminopyridine-3-thiazole as a method for treating or improving rheumatoid arthritis or psoriasis. | [Application]
(1R,3S)-3-aminocyclopentanol hydrochloride is an intermediate in organic synthesis and pharmaceutical intermediate, which can be used in laboratory research and development process and chemical production process. | [Synthesis]
Under nitrogen protection, 82 g of isopropanol was added to the reaction flask. Stirring was turned on, the reaction temperature was controlled at 5 °C, and 93.4 g of pivaloyl chloride was slowly added dropwise. After the dropwise addition, the reaction temperature was adjusted to 25 °C and the esterification reaction was carried out for 30 min. Subsequently, a solution prepared from 52 g of Compound III dissolved in 53 g of isopropanol was added dropwise to the reaction system. After completion of the dropwise addition, the deprotection reaction was carried out at room temperature for 12 h. The reaction process was monitored by GC. At the end of the reaction, the temperature of the reaction system was controlled at 0 °C and stirring was continued for 1 h to ensure complete precipitation of solids. Filtration was carried out under nitrogen protection and the resulting filter cake was washed with isopropanol at 5 °C until the washings were colorless. Next, the filter cake was washed with 40 g of acetone, stirred and heated to 50 °C and maintained for 2 hours. After completion of the reaction, the product was cooled to 0 °C and filtered under nitrogen protection, and the filter cake was washed with acetone at 5 °C until no droplets fell. Finally, the product was dried under vacuum at 40 °C for 12 h to give 25.4 g of (1R,3S)-3-aminocyclopentanol hydrochloride, which was 99.75% pure by GC, with less than 0.01% optical isomer impurities, and 69.8% overall yield based on Compound I. | [References]
[1] Patent: CN108774145, 2018, A. Location in patent: Paragraph 0080; 0083; 0085; 0088; 0089; 0092; 0093; 0096
[2] Patent: CN108774145, 2018, A. Location in patent: Paragraph 0104 |
|
|




![Carbamic acid, [(1S,3R)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167465-99-8.gif)
