| Identification | Back Directory | [Name]
TAS-116 | [CAS]
1260533-36-5 | [Synonyms]
TAS-116
TAS 116;TAS116
Des-iPr-TAS-116
3-ethyl-4-{3-isopropyl-4-(4-(1-methyl-1H-pyrazol-4-yl)-1H-imidazol-1-yl)-1H-pyrazolo[3,4-b]pyridin-1-yl}benzamide
Benzamide, 3-ethyl-4-[3-(1-methylethyl)-4-[4-(1-methyl-1H-pyrazol-4-yl)-1H-imidazol-1-yl]-1H-pyrazolo[3,4-b]pyridin-1-yl]- | [Molecular Formula]
C25H26N8O | [MDL Number]
MFCD28502196 | [MOL File]
1260533-36-5.mol | [Molecular Weight]
454.53 |
| Chemical Properties | Back Directory | [Boiling point ]
661.7±55.0 °C(Predicted) | [density ]
1.36±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO: soluble | [form ]
A solid | [pka]
15.43±0.50(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Description]
TAS-116 (Chinese name: Pimitespib) is an oral heat shock protein 90 (HSP90) α and β subtype selective inhibitor developed by Taiho Pharmaceutical. HSP90 is a molecular chaperone protein that plays a key role in the stability and function of a variety of tumor growth-related proteins. Pimitespib inhibits the activity of HSP90, leading to the degradation of these tumor growth-related proteins, thereby inhibiting the growth and survival of tumor cells. | [Uses]
Pimitespib (TAS-116) is an oral bioavailable, ATP-competitive, highly specific HSP90α/HSP90β inhibitor (Kis of 34.7 nM and 21.3 nM, respectively) without inhibiting other HSP90 family proteins such as GRP94[1]. Pimitespib demonstrates less ocular toxicity[2]. | [Biological Activity]
TAS-116 is a novel small molecule inhibitor of HSP90 with Ki values of 34.7 nmol/L and 21.3 nmol/L for HSP90α and HSP90β, respectively. It does not inhibit other ATPases such as HSP70 (IC50 >200 μmol/L). | [Synthesis]
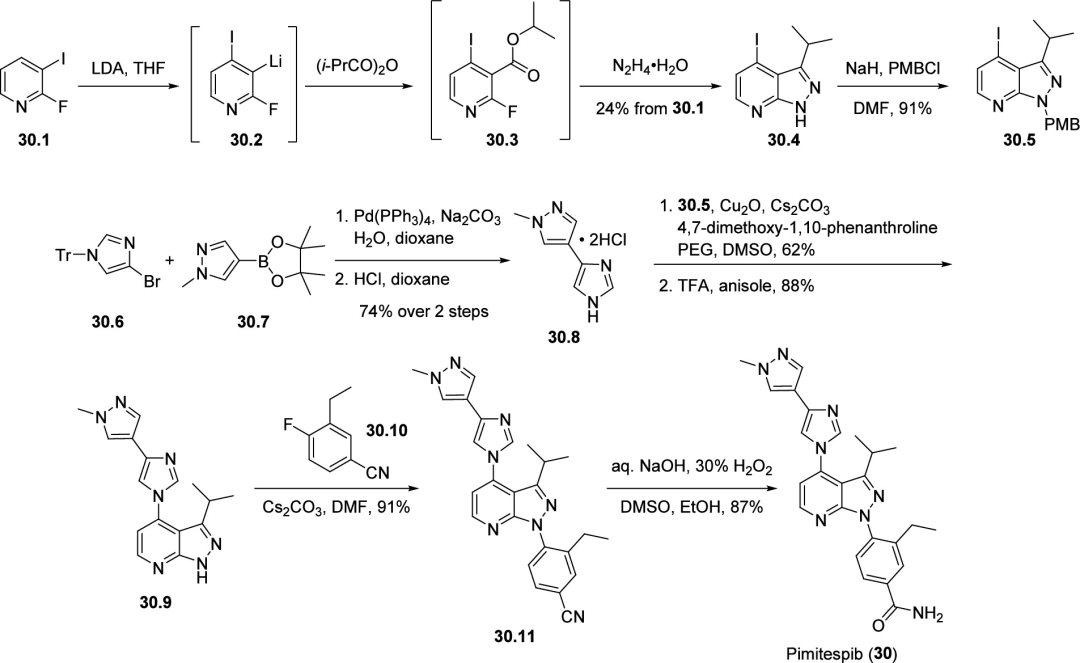
First, pyridine 30.1 was reacted with LDA (dimethyl lithium) to be lithiated at the 3rd position via a halogen dance. The resulting metallated intermediate 30.2 was then quenched with isobutyric anhydride to form pyridine 30.3. Next, nucleophilic substitution reaction (SNAr) with hydrazine hydrate and cyclization gave pyrazolopyridine 30.4 in 24% overall yield from pyridine 30.1. The free nitrogen atom was introduced with a PMB protecting group by reaction with sodium hydride and p-methoxybenzyl chloride to give intermediate 30.5. This intermediate was reacted with 30.8 synthesized by Suzuki coupling via a copper-mediated coupling reaction followed by benzyl deprotection to give 30.9. The PMB protecting group was then removed using trifluoroacetic acid to give 30.9. The final fragment of Pimitespib was introduced by substitution reaction with 30.9 and 30.10. Finally, the nitrile 30.11 was hydrolyzed to the amide using sodium hydroxide and hydrogen peroxide to afford TAS-116 (30).
 | [in vitro]
TAS-116 is a selective inhibitor of HSP90α and HSP90β in the cytoplasm, but has no inhibitory effect on endoplasmic reticulum GRP94 or mitochondrial TRAP1. Treatment of HCT116 cells with 0.3 μM of TAS-1116 for 8 hours resulted in a decrease in DDR1 levels and induction of HSP70. | [in vivo]
In a mouse model with human xenograft tumors, oral administration of TAS-116 induced tumor shrinkage accompanied by depletion of HSP90. In a rat model, TAS-116 was more distributed in subcutaneously xenografted NCI-H1975 non-small cell lung cancer tumors than in the retina. TAS-116 was also active in orthotopically transplanted NCI-H1975 lung tumors. | [target]
| Target | Value | HSP90β
(Cell-free assay) | 21.3 nM(Ki) | HSP90α
(Cell-free assay ) | 34.7 nM(Ki) |
| [IC 50]
HSP90α: 34.7 nM (Ki); HSP90β: 21.3 nM (Ki) | [storage]
Store at -20°C | [References]
[1] Ohkubo S, et al. TAS-116, a highly selective inhibitor of heat shock protein 90α and β, demonstrates potent antitumor activity and minimal ocular toxicity in preclinical models. Mol Cancer Ther. 2015 Jan;14(1):14-22. DOI:10.1158/1535-7163.MCT-14-0219
[2] Suzuki R, et al. Anti-tumor activities of selective HSP90α/β inhibitor, TAS-116, in combination with PS-341 in multiple myeloma. Leukemia. 2015 Feb;29(2):510-4. DOI:10.1038/leu.2014.300
[3] Utsugi T. New challenges and inspired answers for anticancer drug discovery and development. Jpn J Clin Oncol. 2013 Oct;43(10):945-53. DOI:10.1093/jjco/hyt131 |
|
| Company Name: |
NCE Biomedical Co.,Ltd.
|
| Tel: |
4000-027-021 |24 +86-13986109188 | +86-15623472865 | +81-08033611988 |
| Website: |
www.yuhua99.com/ShowSupplierProductsList15748/0_EN.htm |
| Company Name: |
SPIRO PHARMA
|
| Tel: |
|
| Website: |
www.spiropharma.com.cn |
|