| Identification | Back Directory | [Name]
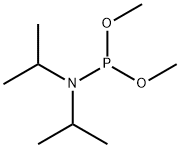
DIMETHYL N,N-DIISOPROPYLPHOSPHORAMIDITE | [CAS]
122194-07-4 | [Synonyms]
DIMETHYL N,N-DIISOPROPYLPHOSPHORAMIDITE
DIMETHYL-N,N-DIISOPROPYLPHOSPHOROAMIDITE
Dimethyl N,N-diisopropylphosphoramidite >=95.0% (GC)
Phosphoramidous acid, N,N-bis(1-methylethyl)-, dimethyl ester | [Molecular Formula]
C8H20NO2P | [MDL Number]
MFCD00153505 | [MOL File]
122194-07-4.mol | [Molecular Weight]
193.22 |
| Chemical Properties | Back Directory | [Boiling point ]
54 °C10 mm Hg(lit.)
| [density ]
0.840 g/mL at 20 °C(lit.)
| [refractive index ]
n20/D 1.420(lit.)
| [Fp ]
100 °C | [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Sparingly), Ethyl Acetate (Sparingly) | [form ]
Oil | [color ]
Clear Colourless | [Stability:]
Moisture Sensitive |
| Hazard Information | Back Directory | [Uses]
A useful reagent for the efficient phosphorylation of alcohols. | [reaction suitability]
reaction type: Phosphorylations | [Synthesis]
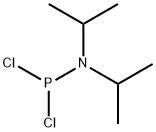
General procedure for the synthesis of dimethyl N,N-diisopropylphosphoramidite amide from methanol and dichloro-N,N-diisopropylphosphoramidite: firstly, the yellow solution obtained from the reaction was subjected to rotary evaporation, followed by the direct addition of 47 mL (325 mmol, 32.9 g) of triethylamine and 250 mL of tetrahydrofuran, and stirring of the mixture to obtain a yellow suspension. Anhydrous methanol 13 mL (322 mmol, 10.3 g) was prepared in a dropping funnel, and methanol was slowly added dropwise to the reaction system over a period of 20 min under nitrogen protection and an ice-salt bath, and the dropping funnel was washed with 150 mL of tetrahydrofuran. Upon completion of the dropwise addition, the reaction mixture was transformed into a white suspension. The ice bath was removed and stirring was continued for 2 h. The reaction was then allowed to stand for a few minutes and diafiltration was performed to obtain a yellow liquid containing a light pink solid. The solid was washed with a small amount of tetrahydrofuran. The tetrahydrofuran was removed by rotary evaporation. The product was dissolved in 10 mL of 5% sodium bicarbonate solution and extracted three times with 20 mL of dichloromethane, the organic phases were combined, the dichloromethane was removed by rotary evaporation, and finally a reduced pressure distillation was performed to collect 78 stable fractions. A colorless liquid and a white solid were observed in the condenser tube, which were identified as the same substance, and the collections could be combined to give a final yield of 16.68 g with 42% yield. | [References]
[1] Journal of the Chemical Society. Perkin Transactions 2, 1998, # 7, p. 1621 - 1628
[2] Patent: CN102977145, 2017, B. Location in patent: Paragraph 0184; 0185 |
|
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
Cool Pharm, Ltd
|
| Tel: |
021-58581007 18019463053 |
| Website: |
www.coolpharm.com.cn |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|