| Identification | Back Directory | [Name]
6-Bromo-1H-pyrazolo[4,3-b]pyridine | [CAS]
1150617-54-1 | [Synonyms]
6-broMo-1H-pyrazolo[4
3-Cya-4-nitro-1H-indazole
6-Bromo-1H-pyrazolo[4,3-b...
6-Bromo-1H-pyrazolo[4,3-b]pyridine
1H-Pyrazolo[4,3-b]pyridine, 6-broMo- | [Molecular Formula]
C6H4BrN3 | [MDL Number]
MFCD12024534 | [MOL File]
1150617-54-1.mol | [Molecular Weight]
198.02 |
| Chemical Properties | Back Directory | [Boiling point ]
329.0±22.0 °C(Predicted) | [density ]
1.894 | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
solid | [pka]
9.72±0.40(Predicted) | [Appearance]
Light yellow to yellow Solid | [InChI]
InChI=1S/C6H4BrN3/c7-4-1-5-6(8-2-4)3-9-10-5/h1-3H,(H,9,10) | [InChIKey]
FONNZZMIJPHSJP-UHFFFAOYSA-N | [SMILES]
C12C=NNC1=CC(Br)=CN=2 |
| Hazard Information | Back Directory | [Uses]
6-Bromo-1H-pyrazolo[4,3-B]pyridine is widely used in the fields of medicine and pesticides.
| [Preparation]
6-Hydrazino-1H-pyrazolo[4,3-B]pyridine can be prepared by reacting 3,6-dichloro-2-pyridinecarboxaldehyde and hydrazine hydrate as raw materials.
| [Synthesis]
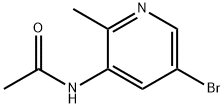
General procedure for the synthesis of 6-bromo-1H-pyrazolo[4,3-b]pyridine from N-(5-bromo-2-methylpyridin-3-yl)acetamide: N-(5-bromo-2-methylpyridin-3-yl)acetamide (8.28 g, 36 mmol) was dissolved in chloroform (550 mL) at room temperature, and potassium acetate (4.32 g, 43.6 mmol) was added sequentially, acetic acid (2.5 mL, 43.6 mmol) and acetic anhydride (6.86 mL, 72.6 mmol). The reaction mixture was stirred at room temperature for 15 min and then warmed up to 40 °C. Subsequently, isoamyl nitrite was slowly added dropwise. After the dropwise addition was completed, the reaction mixture was continued to be stirred. The reaction mixture was cooled to 0 °C and slowly poured into saturated sodium bicarbonate solution (500 mL). The organic phase was separated and the aqueous phase was extracted with dichloromethane (500 mL). The organic phases were combined and concentrated to give a brown oil. The oil was dissolved in methanol (500 mL), cooled to 0°C, and 2M aqueous sodium hydroxide solution (500 mL) was added. The mixture was stirred at room temperature for 1 hour and then the methanol was removed under reduced pressure. The aqueous phase was extracted with ethyl acetate (3 x 500 mL). The organic phases were combined, dried with magnesium sulfate, and the solvent was concentrated under reduced pressure to give 6-bromo-1H-pyrazolo[4,3-b]pyridine (5.5 g, 27.9 mmol, 77% yield) as a light brown solid.1H NMR (400 MHz, CD3OD): δ 8.55 (s, 1H), 8.24 (s, 1H), 8.21 (s, 1H). | [References]
[1] Patent: US2011/111046, 2011, A1. Location in patent: Page/Page column 16
[2] Patent: WO2011/58473, 2011, A1. Location in patent: Page/Page column 33; 34
[3] Patent: US2012/108619, 2012, A1. Location in patent: Page/Page column 30
[4] Patent: WO2014/26327, 2014, A1. Location in patent: Page/Page column 89
[5] Patent: WO2014/28589, 2014, A2. Location in patent: Page/Page column 108; 109 |
|
|