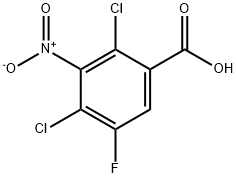
| Identification | More | [Name]
2,4-Dichloro-5-fluoro-3-nitrobenzoic acid | [CAS]
106809-14-7 | [Synonyms]
2,4-DICHLORO-3-NITRO-5-FLUORO BENZOIC ACID
2,4-DICHLORO-5-FLUORO-3-NITROBENZOIC ACID
TIMTEC-BB SBB003130
2,4-difluoro-5-fluoro-3-nitro benzoic acid
2,4-Dichloro-3-nitro-5-fluorob
2,4-Dichloro-3-hitro-5-fluorobenzoicacid | [Molecular Formula]
C7H2Cl2FNO4 | [MDL Number]
MFCD03427137 | [Molecular Weight]
254 | [MOL File]
106809-14-7.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29163990 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powder | [Uses]
2,4-Dichloro-5-fluoro-3-nitrobenzoic acid may be used to synthesize:
- ethyl 3-(N,N-dimethylamino)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl)acrylate
- 7-chloro-1-cyclopropyl-6-fluoro-8-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
- ethyl-3-(N,N-dimethylamino)-2-(2,4-dichloro-5-fluoro-3-nitrobenzoyl) acrylate
- substituted 4(1H)-oxoquinoline-3-carboxylic acid
| [Synthesis Reference(s)]
Journal of Heterocyclic Chemistry, 25, p. 927, 1988 DOI: 10.1002/jhet.5570250343 | [General Description]
2,4-Dichloro-5-fluoro-3-nitrobenzoic acid can be prepared from 2,4-dichloro-5-fluorobenzoic acid. | [Synthesis]
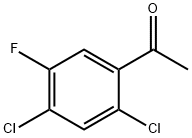
General procedure for the synthesis of 2,4-dichloro-5-fluoro-3-nitrobenzoic acid from 2',4'-dichloro-5'-fluoroacetophenone: 55 g (0.55 mol) of concentrated sulfuric acid was added to a 250 mL three-necked flask with a stirrer and reflux condenser tube. Under stirring (stirring speed of 400 r/min), 19.29 g (0.3 mol) fuming nitric acid was slowly added to the three-necked flask. Subsequently, the reaction mixture was warmed up to 103 °C and 10.46 g (0.05 mol) of pre-melted 2,4-dichloro-5-fluoroacetophenone was slowly added dropwise at this temperature, controlling the dropwise temperature to be between 103 and 110 °C. After the dropwise addition was completed, the reaction temperature was maintained at 106 °C to continue the reaction for 3 hours. After completion of the reaction, the mixture was cooled to 10 °C and separated by filtration. The filtrate was recovered and set aside, and the filter cake was washed with water. Finally, the filter cake was recrystallized with toluene to give 11.67 g of 2,4-dichloro-5-fluoro-3-nitrobenzoic acid with 99% purity and 91% yield. | [References]
[1] Patent: CN103922942, 2016, B. Location in patent: Paragraph 0026; 0027
[2] Journal of Heterocyclic Chemistry, 1988, vol. 25, p. 927 - 930 |
|
|