| Identification | More | [Name]
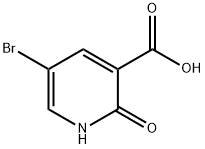
5-Bromo-2-hydroxynicotinic acid | [CAS]
104612-36-4 | [Synonyms]
5-BROMO-2-HYDROXY-3-PYRIDINECARBOXYLIC ACID
5-BROMO-2-HYDROXYNICOTINIC ACID
5-BROMO-2-HYDROXYPYRIDINE-3-CARBOXYLIC ACID
5-BROMO-3-CARBOXY-2(1H)-PYRIDINONE
5-Bromo-2-hydroxynicotinic acid 98%
5-BROMO-2-HYDROXYNICOTINIC ACID HPLC 99%
2-Hydroxy-5-bromonicotinic acid
3-Pyridinecarboxylic acid, 5-bromo-2-hydroxy-
5-Bromo-2-hydroxynicotinic acid ,98% | [EINECS(EC#)]
692-269-1 | [Molecular Formula]
C6H4BrNO3 | [MDL Number]
MFCD07363801 | [Molecular Weight]
218 | [MOL File]
104612-36-4.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal | [Melting point ]
287°C | [Boiling point ]
354.7±42.0 °C(Predicted) | [density ]
2.015±0.06 g/cm3(Predicted) | [Fp ]
287°C | [storage temp. ]
Keep Cold | [form ]
powder to crystal | [pka]
2.09±0.20(Predicted) | [color ]
Light orange to Yellow to Green | [Decomposition ]
287 ºC | [Detection Methods]
HPLC,NMR | [InChI]
InChI=1S/C6H4BrNO3/c7-3-1-4(6(10)11)5(9)8-2-3/h1-2H,(H,8,9)(H,10,11) | [InChIKey]
GYXOTADLHQJPIP-UHFFFAOYSA-N | [SMILES]
C1(=O)NC=C(Br)C=C1C(O)=O | [CAS DataBase Reference]
104612-36-4(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal | [Synthesis Reference(s)]
Synthetic Communications, 19, p. 553, 1989 DOI: 10.1080/00397918908050699
Synthesis, p. 528, 2002 DOI: 10.1055/s-2002-20959 | [Synthesis]
General procedure for the synthesis of 5-bromopyridine-3-carboxylic acid-2-one from 2-hydroxynicotinic acid: the compound was prepared according to the method of Y.S. Lo (Synthetic Communications, 1989, 553). Bromine (0.77 eq.) was slowly added dropwise to a stirred solution of 50% NaOH (2.4 eq.) in water (1 M solution) at 0 °C. After 5 min, 50% NaOH (3 eq.) was appended to the mixture, followed by the addition of solid 2-hydroxynicotinic acid (1 eq.) and the resultant solution was stirred continuously at 50 °C. After 20 h, a mixture of bromine ( 0.38 eq.) and a pre-prepared solution of 50% NaOH (1.2 eq.) in water (1 M solution) and continued stirring at 50 °C for 24 hours. Upon completion of the reaction, the mixture was cooled to 0 °C and acidified to pH 2 with concentrated hydrochloric acid to precipitate the solid product. The solid was separated by filtration, washed sequentially with warm water/isopropanol (3:1) and ether, and dried to give 5-bromo-2-hydroxynicotinic acid in 87% yield as an off-white solid. The product characterization data were as follows: 1H NMR (400 MHz, DMSO) δ 8.25 (1H, d, J = 2.7 Hz), 8.33 (1H, d, J = 2.7 Hz), 13.84 (2H, bs); 13C NMR (400 MHz, DMSO) δ 99.45, 117.97, 142.01, 147.42, 163.22, 163.88; MS (ES-) m/z 218-216 (M-H)-. | [References]
[1] Patent: WO2004/110442, 2004, A1. Location in patent: Page 17; 18
[2] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 9, p. 2566 - 2569
[3] Patent: US2002/161000, 2002, A1
[4] Patent: US2006/287292, 2006, A1. Location in patent: Page/Page column 26
[5] Organic Process Research and Development, 2008, vol. 12, # 4, p. 603 - 613 |
|
|