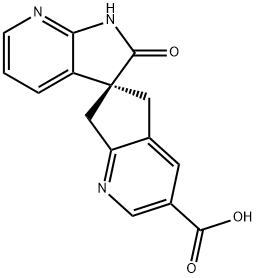
Ubrogepant Intermediate synthesis
- Product Name:Ubrogepant Intermediate
- CAS Number:1375541-21-1
- Molecular formula:C15H11N3O3
- Molecular Weight:281.27
![Spiro[6H-cyclopenta[b]pyridine-6,3'-[3H]pyrrolo[2,3-b]pyridine]-3-carboxylic acid, 1',2',5,7-tetrahydro-2'-oxo-, methyl ester, (3'S)-](/CAS/20210111/GIF/1375541-24-4.gif)
1375541-24-4
5 suppliers
inquiry

1375541-21-1
70 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: methyl (6S)-2'-oxo-1',2’,5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxylatewith sodium hydroxide in methanol; for 1 h;Reflux;
Stage #2: with hydrogenchloride in methanol;water at 23; pH=~ 6;
Steps:
I.C
Step C: (6S)-2'-Oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxylic acidA mixture of methyl (6S)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxylate (30.0 g, 102 mmol) and aqueous 6 N sodium hydroxide solution (50.8 mL, 305 mmol) in MeOH (920 mL) was heated at reflux for 1 h. The mixture was allowed to cool to 23° C. before it was acidified to pH6 with aqueous 1 N hydrochloric acid solution, resulting in a black precipitate which was removed by filtration. The filtrate was concentrated under reduced pressure to a volume of 100 mL and then partitioned between water (500 mL) and 2-methyltetrahydrofuran (2-MeTHF, 250 mL). The aqueous layer was extracted with 2-MeTHF (5×250 mL), and the combined organic layers were dried over sodium sulfate and concentrated to provide the title compound. MS: m/z=282.0 (M+1).
References:
US2012/122899,2012,A1 Location in patent:Page/Page column 18
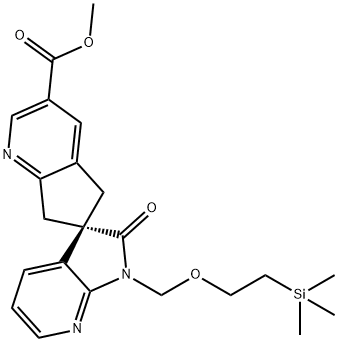
1375542-65-6
5 suppliers
inquiry

1375541-21-1
70 suppliers
inquiry
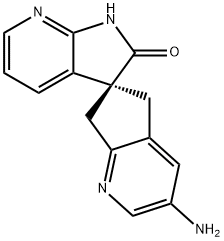
1059186-43-4
4 suppliers
inquiry

1375541-21-1
70 suppliers
inquiry
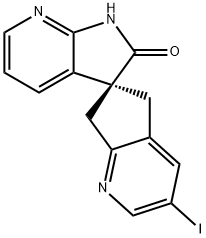
1189570-15-7
3 suppliers
inquiry

1375541-21-1
70 suppliers
inquiry
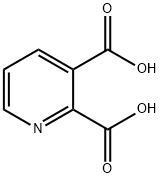
89-00-9
487 suppliers
$5.00/5g

1375541-21-1
70 suppliers
inquiry