
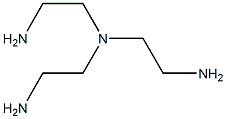
TRIS(2-DIMETHYLAMINOETHYL)AMINE synthesis
- Product Name:TRIS(2-DIMETHYLAMINOETHYL)AMINE
- CAS Number:33527-91-2
- Molecular formula:C12H30N4
- Molecular Weight:230.39
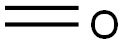
50-00-0
14350-52-8

33527-91-2
Me6TREN was prepared according to the method reported in the literature [52]. The procedure was as follows: first, 30 mL of a methanol solution of 3.0 M HCl was slowly added dropwise to a 50 mL methanol solution containing 4.0 mL (0.027 mol) of tris(2-aminoethyl)amine. After the reaction mixture was stirred at room temperature for 1 hour, the precipitate was collected by filtration and washed three times with 50 mL of methanol to give 6.72 g (0.026 mol) of ClNH3CH2CH2)3NHCl in 98% yield. Subsequently, 6.72 g of ClNH3CH2CH2)3NHCl, 10 mL of distilled water, 50 mL of formic acid and 46 mL of aqueous formaldehyde were mixed. The mixture was heated in an oil bath at 120°C with stirring for 6 hours. Upon completion of the reaction, the volatile components were removed by rotary evaporation. To the solid residue was added 100 mL of a 10 wt% aqueous NaOH solution. The resulting aqueous phase was extracted four times with 100 mL of ether. The organic layers were combined, dried with anhydrous NaOH and concentrated by rotary evaporation. Finally, 6.0 g of colorless oily product was obtained in 89% yield by vacuum distillation at 62 °C. The structure of the product was confirmed by 1H NMR (CDCl3, 400 MHz): δ 2.20 (s, 18H, CH3); δ 2.3-2.37 (m, 6H, CH2); δ 2.56-2.60 (m, 6H, CH2).
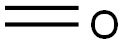
50-00-0
892 suppliers
$10.00/25g
64-18-6
1126 suppliers
$22.12/250ML

4097-89-6
192 suppliers
$10.00/1g

33527-91-2
112 suppliers
$8.00/250mg
Yield:33527-91-2 26%
Reaction Conditions:
in water at 100;Cooling with ice;Inert atmosphere;
Steps:
Synthesis of Tris(2-(dimethylamino)ethyl)amine (Me6TREN)
Synthesis of Tris(2-(dimethylamino)ethyl)amine (Me6TREN)
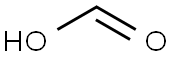
In a 250 mL round bottom flask TREN (10.00 g, 0.07 mol) in water (25 mL) was added dropwise using a pressure equalizer to an ice chilled mixture of formaldehyde (36% in water) (39.00 g, 0.47 mol) and formic acid (55.16 g, 1.2 mol).
After complete addition of TREN, the RB was brought to an oil bath and the solution was refluxed gently overnight at 100° C.
It was cooled to room temperature and water was removed by trap-to-trap distillation.
To remove unreacted formic acid, product was dissolved in 20 mL of acetonitrile and passed through a basic alumina column.
Acetonitrile was removed by rotary evaporation and product was further purified by vacuum distillation. Yield=4.10 g (26%).
1H-NMR (CDCl3, 7.27 ppm): 2.22 (s, -CH3), 2.37 (dd, -CH2N(CH2)2), 2.60 (dd, -CH2N(CH3)2).
13C-NMR (CDCl3, 77.23 ppm): 46.1 (-CH3), 53.3 (-CH2N(CH2)2), 57.7 (-CH2N(CH3)2).
References:
The University of Akron;Pugh, Coleen R.;Singh, Anirudha US8524942, 2013, B2 Location in patent:Page/Page column 21
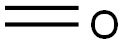
50-00-0
892 suppliers
$10.00/25g

4097-89-6
192 suppliers
$10.00/1g

33527-91-2
112 suppliers
$8.00/250mg

64-18-6
1126 suppliers
$22.12/250ML

4097-89-6
192 suppliers
$10.00/1g

33527-91-2
112 suppliers
$8.00/250mg

555-77-1
0 suppliers
inquiry
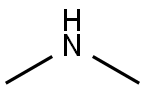
124-40-3
552 suppliers
$18.00/100ml

33527-91-2
112 suppliers
$8.00/250mg