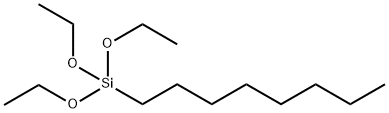
Triethoxyoctylsilane synthesis
- Product Name:Triethoxyoctylsilane
- CAS Number:2943-75-1
- Molecular formula:C14H32O3Si
- Molecular Weight:276.49

111-66-0

998-30-1
78-10-4

2943-75-1
The typical experimental procedure (Table 2, entry 1) was as follows: Ni(acac)2 (1a) (1.3 mg, 0.005 mmol) was dissolved in THF (5 mL) at room temperature, and 1-octene (112 mg, 1.0 mmol) and triethoxysilane (164 mg, 1.0 mmol) were added sequentially under stirring. After stirring the mixed solution for 1 min, NaBHEt3 (1.0 M in THF, 5 mL, 0.005 mmol) was added and the reaction mixture was subsequently heated to 50 °C. This temperature was maintained and the reaction progress was monitored by gas chromatography (GLC). Upon completion of the reaction, homotrimethylbenzene (60 mg, 0.50 mmol) was added to the mixture as an internal standard.GLC analysis showed the products to be n-octyltriethoxysilane (0.90 mmol, 90%) and tetraethyl silicate (0.05 mmol, 5%). The reaction solution was concentrated under vacuum and the residue was purified by gel permeation chromatography (GPC) using toluene as eluent to give n-octyltriethoxysilane (234 mg, 0.85 mmol, 85%). The 1H, 13C{1H} and 29Si{1H} NMR spectra of the isolated compounds were in agreement with the data reported in the literature. Similar methods were applied to the hydrogenosilylation of other silanes with 1,3-dienes/olefins/alkynes, except for the reactions, which were carried out at room temperature (Table 2, entries 2-3). The 1H/13C NMR spectral data of the new compounds are detailed in the Supplementary Material.

111-66-0
173 suppliers
$10.00/10ml

998-30-1
252 suppliers
$30.00/25g

2943-75-1
343 suppliers
$8.00/25g
Yield:2943-75-1 100%
Reaction Conditions:
at 90; for 5 h;Inert atmosphere;
Steps:
11 Example 11
In a 500-ml three-necked flask, octene (1.25 mol) was added, and Catalyst 1 (1.25 mmol) prepared in Example 1 was slowly heated to 90°C under a nitrogen atmosphere.While stirring, triethoxyhydrosilane (1.5 mol) was added dropwise through the dropping funnel. The dropwise addition time was 0.5 hours, the reaction temperature was maintained, and the stirring reaction was continued for 5 hours.After cooling to room temperature, the corresponding fractions were collected by distillation under reduced pressure. The conversion of octene was 94.7% as determined by GC-MS.The yield of the β adduct 1-triethoxysilyloctane was 100%.
References:
CN107857776,2018,A Location in patent:Paragraph 0048; 0049

111-66-0
173 suppliers
$10.00/10ml

998-30-1
252 suppliers
$30.00/25g

78-10-4
438 suppliers
$12.00/25g

2943-75-1
343 suppliers
$8.00/25g

111-66-0
173 suppliers
$10.00/10ml

998-30-1
252 suppliers
$30.00/25g

78-10-4
438 suppliers
$12.00/25g
2157-42-8
6 suppliers
inquiry

2943-75-1
343 suppliers
$8.00/25g

111-66-0
173 suppliers
$10.00/10ml

998-30-1
252 suppliers
$30.00/25g

2943-75-1
343 suppliers
$8.00/25g
911808-73-6
0 suppliers
inquiry

111-66-0
173 suppliers
$10.00/10ml

998-30-1
252 suppliers
$30.00/25g
111-65-9
285 suppliers
$12.00/25g

2943-75-1
343 suppliers
$8.00/25g