
THIOMORPHOLINE 1,1-DIOXIDE HYDROCHLORIDE synthesis
- Product Name:THIOMORPHOLINE 1,1-DIOXIDE HYDROCHLORIDE
- CAS Number:59801-62-6
- Molecular formula:C4H10ClNO2S
- Molecular Weight:171.65
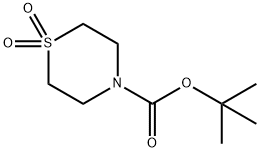
215791-95-0

59801-62-6
The general procedure for the synthesis of thiomorpholine-1,1-dioxide hydrochloride from tert-butyl thiomorpholine-4-carboxylate 1,1-dioxide was as follows: tert-butyl 1,1-dioxothiomorpholine-4-carboxylate (2.03 g, 8.63 mmol) was dissolved in a hydrochloric acid-methanol (10%, 20 mL, purchased from Tokyo Kasei Kogyo Co.) and tetrahydrofuran (20 mL) in a solvent mixture. Concentrated hydrochloric acid (4.0 mL) was slowly added to the reaction system under stirring at room temperature. The reaction mixture was continued to be stirred at room temperature for 3 hours. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure. Methanol (20 mL), tetrahydrofuran (20 mL), and concentrated hydrochloric acid (4.0 mL) were added to the resulting solid, followed by the addition of water (10 mL) to ensure complete dissolution of the solid. This solution was stirred at room temperature for 1 hour. The solvent was again concentrated under reduced pressure to remove the solvent. The resulting crystals were suspended in methanol, filtered, washed with methanol and dried under ventilated conditions to give thiomorpholine-1,1-dioxide hydrochloride as colorless crystals (1.49 g, 8.65 mmol, quantitative yield). The product was characterized by 1H-NMR (DMSO-d6): δ 3.54 (8H, m), 9.83 (2H, brs).

215791-95-0
11 suppliers
inquiry

59801-62-6
201 suppliers
$6.00/1g
Yield:59801-62-6 100%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;methanol;water at 20; for 5 h;
Steps:
54-3 Production example 54-3; Thiomorpholine 1,1-dioxide monohydrochloride
tert-Butyl 1,1-dioxothiomorpholine-4-carboxylate (2.03 g, 8.63 mmol) was dissolved in a mixture of hydrochloric acid-methanol 10 (20 ml, purchased from Tokyo Kasei Kogyo Co., Ltd) and tetrahydrofuran (20 ml); hydrochloric acid (4.0 ml) was added thereto during stirring at room temperature; and the reaction mixture was stirred at room temperature for 3 hours. The reaction mixture was concentrated; methanol (20 ml), tetrahydrofuran (20 ml) and hydrochloric acid (4.0 ml) were added to the obtained crystals. Furthermore, water (10 ml) was added to this solution to perfectly dissolve the crystals; and this solution was stirred at room temperature for 1 hour. The solvent was concentrated under reduced pressure; and the obtained crystals were suspended in methanol, filtered off, washed with methanol, and dried under aeration to yield the title compound as colorless crystals (1.49 g, 8.65 mmol, quantitative). 1H-NMR Spectrum (DMSO-d6) δ (ppm): 3.54 (8H, m), 9.83 (2H, brs).
References:
EP1522540,2005,A1 Location in patent:Page/Page column 70
25343-91-3
31 suppliers
$40.10/1g
627-11-2
238 suppliers
$33.00/25g

59801-62-6
201 suppliers
$6.00/1g