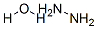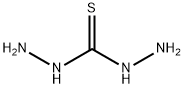
Thiocarbohydrazide synthesis
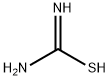
- Product Name:Thiocarbohydrazide
- CAS Number:2231-57-4
- Molecular formula:CH6N4S
- Molecular Weight:106.15
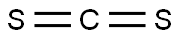
75-15-0

2231-57-4
The general procedure for the synthesis of thiocarbazide from carbon disulfide is as follows: in a 50 mL round-bottomed flask equipped with a magnetic stirrer, carbon disulfide (5 mmol) and the corresponding amine (10 mmol) were added sequentially in an ice-bath under cooled conditions, followed by water (5 mL). The flask was immediately sealed. The reaction mixture was stirred vigorously at room temperature. The progress of the reaction was monitored by thin layer chromatography (TLC) with the unfolding agent being 10% methanol:chloroform or ethyl acetate:hexane. Upon completion of the reaction, for most cases, the aqueous phase was removed by distillation under reduced pressure on a rotary evaporator to give a solid product; for the reactions of entries 7, 9, 10, 11, 14, and 15, the reaction mixture would solidify directly upon completion of the reaction. Ultimately, the resulting solid product is purified by recrystallization from ethanol or by washing with a solvent mixture of ethyl acetate:hexane.
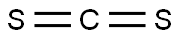
75-15-0
267 suppliers
$40.00/100 g

2231-57-4
232 suppliers
$31.00/5g
Yield:2231-57-4 98%
Reaction Conditions:
with hydrazine in water at 20; for 0.333333 h;Green chemistry;
Steps:
Typical experimental procedure for symmetrical thioureas derivatives
General procedure: To a 50 mL round bottom flask equipped with a magnetic stirrer, add carbon disulphide (5 mmol) and amine (10 mmol) in ice cold condition followed by addition of water (5 mL). Cork the flask. The reaction mixture was stirred vigorously at room temperature. The progress of the reaction was checked by TLC (10% MeOH: chloroform or EtOAc: hexane). After completion of reaction, solid was obtained by removal of water under reduced pressure on rotary evaporator while in some cases (Entry 7, 9, 10, 11 and 14, 15) the reaction mixture solidified when the reaction on completion. The obtained solid product was recrystallized with ethanol or washing with mixture EtOAc : hexane.
References:
Jangale, Asha D.;Kumavat, Priyanka P.;Wagh, Yogesh B.;Tayade, Yogesh A.;Mahulikar, Pramod P.;Dalal, Dipak S. [Synthetic Communications,2015,vol. 45,# 3,p. 376 - 385] Location in patent:supporting information
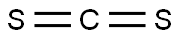
75-15-0
267 suppliers
$40.00/100 g
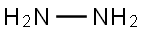
302-01-2
106 suppliers
$39.89/5g

2231-57-4
232 suppliers
$31.00/5g
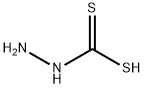
471-32-9
3 suppliers
inquiry

2231-57-4
232 suppliers
$31.00/5g