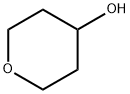
Tetrahydro-4-pyranol synthesis
- Product Name:Tetrahydro-4-pyranol
- CAS Number:2081-44-9
- Molecular formula:C5H10O2
- Molecular Weight:102.13
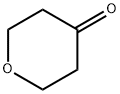
29943-42-8

2081-44-9
General procedure for the synthesis of tetrahydropyran-4-ol from tetrahydropyranone: To a tetrahydrofuran (THF, 25 mL) solution of tetrahydropyranone (5.0 g, 50 mmol), which was cooled to 0 °C, a THF (50 mL) suspension of lithium aluminum hydride (LiAlH4, 3.8 g, 0.1 mol) was slowly added. The reaction mixture was stirred at 0 °C for 30 min, followed by sequential addition of water (3.8 mL), 15% aqueous sodium hydroxide (NaOH) solution (3.8 mL) and water (11.4 mL) to quench the reaction. The mixture was filtered and the solid was washed with ethyl acetate (70 mL x 2). The filtrates were combined and evaporated to give tetrahydropyran-4-ol (5.09 g, 99% yield).

29943-42-8
429 suppliers
$5.00/1g

2081-44-9
293 suppliers
$6.00/1g
Yield:2081-44-9 99%
Reaction Conditions:
Stage #1: dihydro-2H-pyran-4(3H)-onewith lithium aluminium hydride in tetrahydrofuran at 0; for 0.5 h;
Stage #2: with sodium hydroxide in tetrahydrofuran;lithium hydroxide monohydrate;
Steps:
8.1
To a solution of 8a (5.Og, 50mmol) in THF (25mL) cooled to O0C, was added a suspension Of LiAlH4 (3.8g, O.lmol) in THF (5OmL) slowly. The resulting mixture was stirred at O0C for 30min, then was added water (3.8mL), followed by aqueous 15% NaOH (3.8mL) and water (1 1.4mL). The mixture was filtered and the solid was washed with ethyl ester (70mLχ2). The combined filtrate was evaporated to afford 8b (5.09g, 99%).
References:
WO2008/88881,2008,A1 Location in patent:Page/Page column 39

67-56-1
782 suppliers
$9.00/25ml
187737-37-7
1 suppliers
inquiry
1120-97-4
85 suppliers
$21.00/5g

2081-44-9
293 suppliers
$6.00/1g
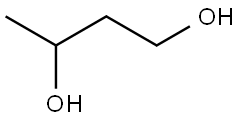
107-88-0
590 suppliers
$6.00/100g
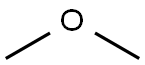
115-10-6
106 suppliers
$75.00/25g
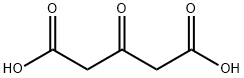
542-05-2
497 suppliers
$6.00/10g

2081-44-9
293 suppliers
$6.00/1g
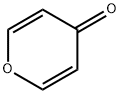
108-97-4
81 suppliers
$61.00/250mg

2081-44-9
293 suppliers
$6.00/1g

72548-31-3
1 suppliers
inquiry

2081-44-9
293 suppliers
$6.00/1g